Abstract
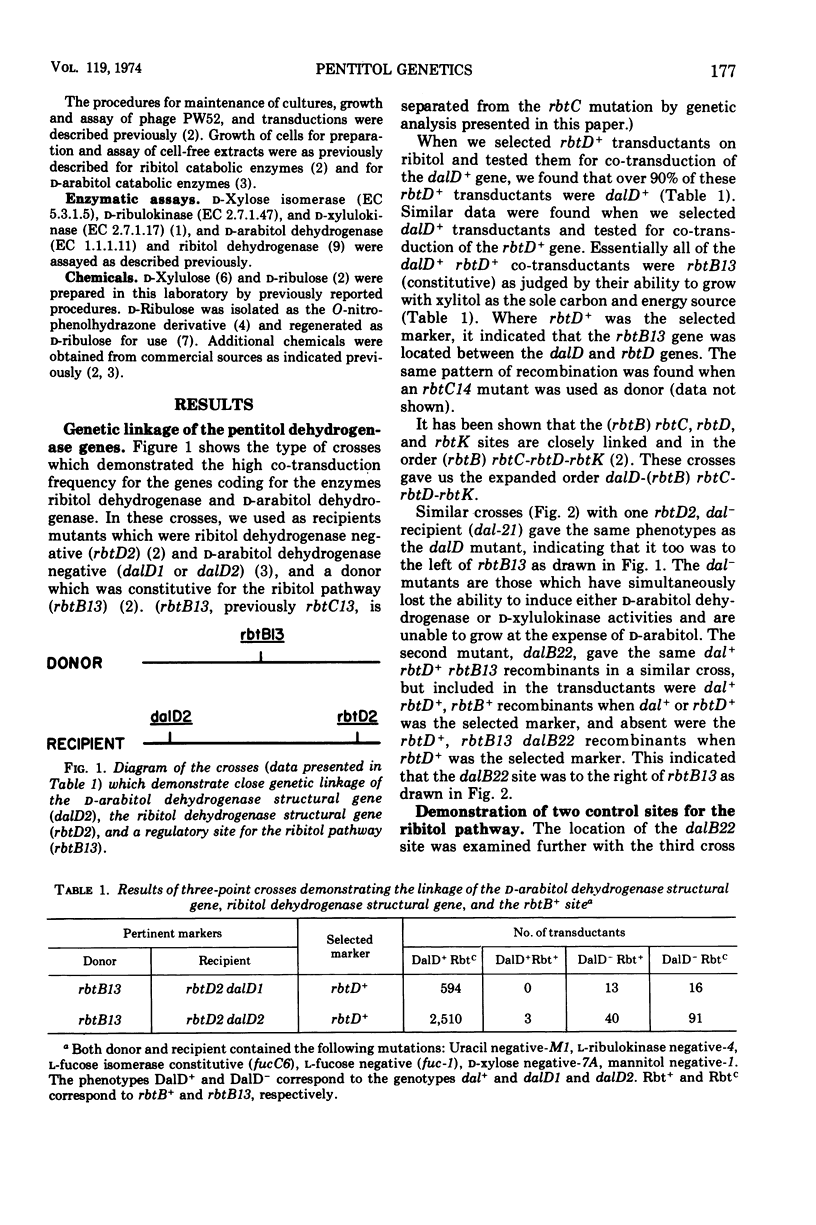
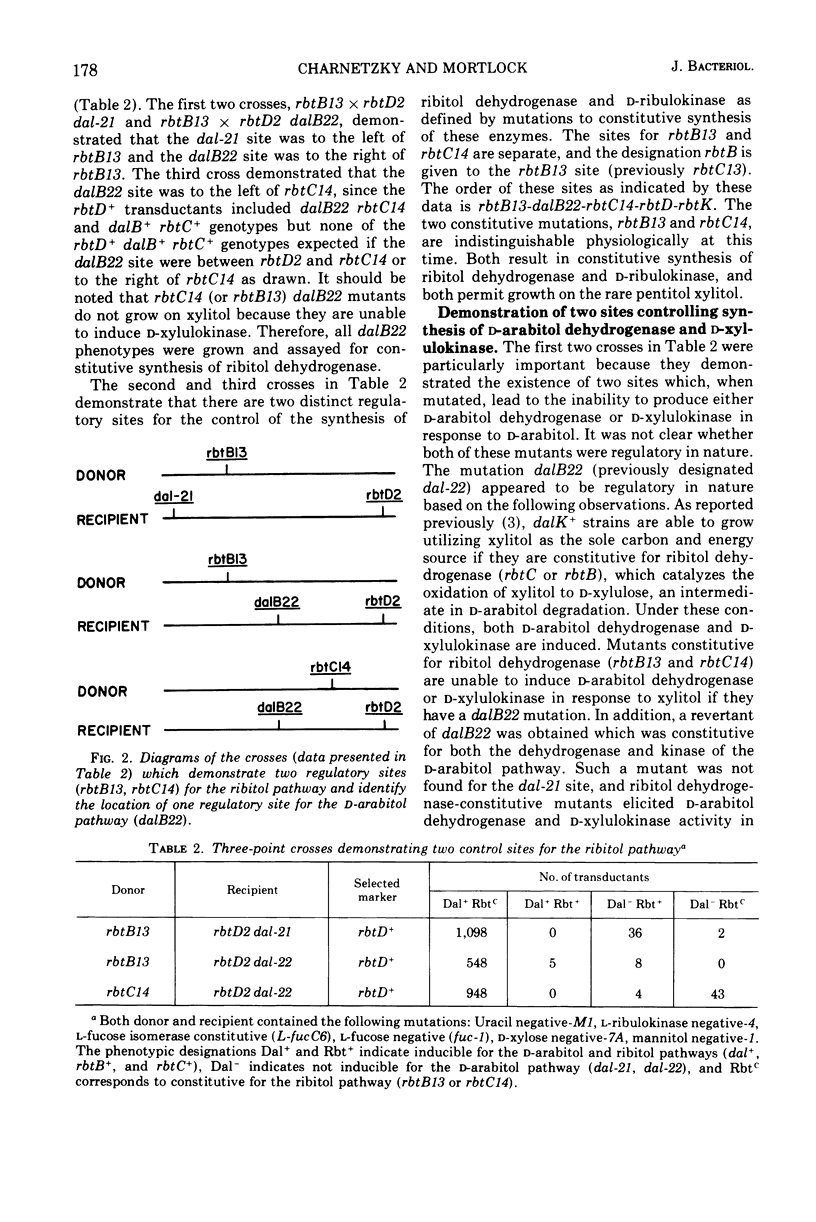
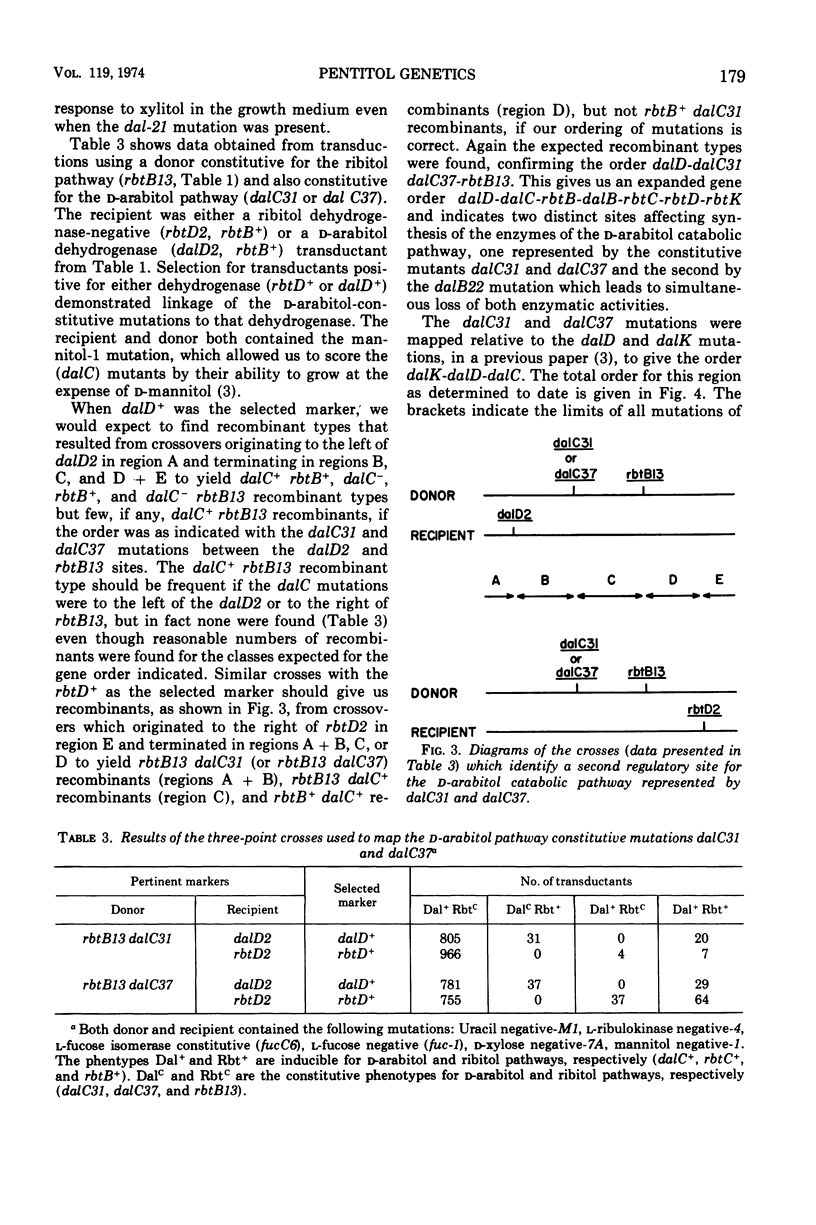
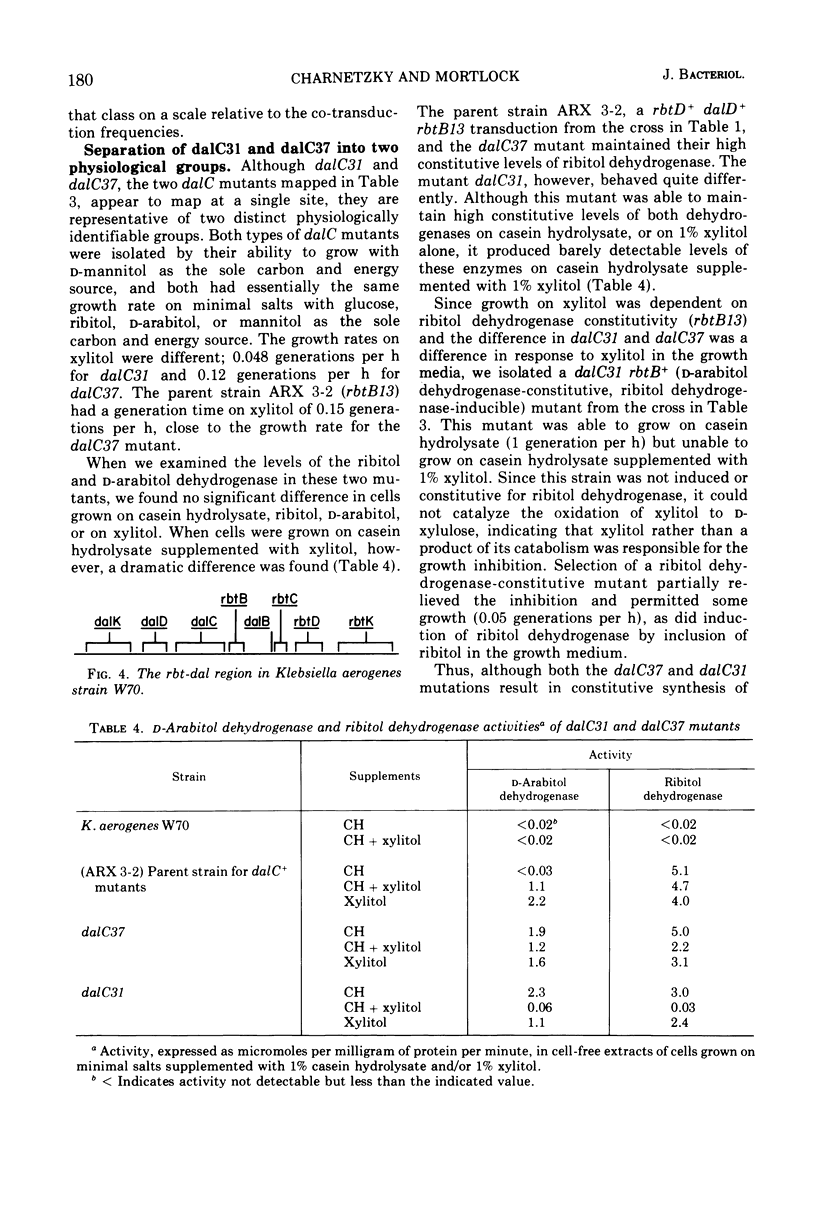
Klebsiella aerogenes strain W70 has separate inducible pathways for the degradation of the pentitols ribitol and d-arabitol. These pathways are closely linked genetically as determined by transduction with phage PW52. There are two regulatory sites for the ribitol catabolic pathway as defined by loci for mutations to constitutive synthesis of ribitol dehydrogenase and d-ribulokinase, rbtB and rbtC. The two control sites are separated by a site represented by the dalB22 mutation. This mutation deprives the cell of the ability to induce synthesis of d-arabitol dehydrogenase and d-xylulokinase activities. Two additional regulatory mutations for the d-arabitol pathway, dalC31 and dalC37, map to the opposite side of rbtB13 relative to dalB22. The order of the genetic sites thus far determined for this region is dalK-dalD-dalC31, dalC37-rbtB13-dalB22-rbtC14-rbtD-rbtK, where dalK and dalD represent structural genes for the kinase and dehydrogenase of the d-arabitol pathway, respectively, and rbtK and rbtD represent the corresponding genes for the ribitol pathway. The two mutations that lead to constitutive synthesis of the d-arabitol-induced enzymes, dalC31 and dalC37, have different phenotypes with regard to their response to xylitol. The growth of dalC31 is inhibited by xylitol, but the toxicity can be reduced by increasing the levels of ribitol dehydrogenase either by induction with ribitol or by selection of a ribitol dehydrogenase-constitutive mutation.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ANDERSON R. L., WOOD W. A. Purification and properties of L-xylulokinase. J Biol Chem. 1962 Apr;237:1029–1033. [PubMed] [Google Scholar]
- COHEN S. Studies on D-ribulose and its enzymatic conversion to D-arabinose. J Biol Chem. 1953 Mar;201(1):71–84. [PubMed] [Google Scholar]
- Charnetzky W. T., Mortlock R. P. D-Arabitol catabolic pathway in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):170–175. doi: 10.1128/jb.119.1.170-175.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charnetzky W. T., Mortlock R. P. Ribitol catabolic pathway in Klebsiella aerogenes. J Bacteriol. 1974 Jul;119(1):162–169. doi: 10.1128/jb.119.1.162-169.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPhee D. G., Sutherland I. W., Wilkinson J. F. Transduction in Klebsiella. Nature. 1969 Feb 1;221(5179):475–476. doi: 10.1038/221475a0. [DOI] [PubMed] [Google Scholar]
- Oliver E. J., Bisson T. M., LeBlanc D. J., Mortlock R. P. D-Ribulose production by a mutant of Aerobacter aerogens. Anal Biochem. 1969 Feb;27(2):300–305. doi: 10.1016/0003-2697(69)90036-0. [DOI] [PubMed] [Google Scholar]
- WOOD W. A., McDONOUGH M. J., JACOBS L. B. Rihitol and D-arabitol utilization by Aerobacter aerogenes. J Biol Chem. 1961 Aug;236:2190–2195. [PubMed] [Google Scholar]


