Abstract
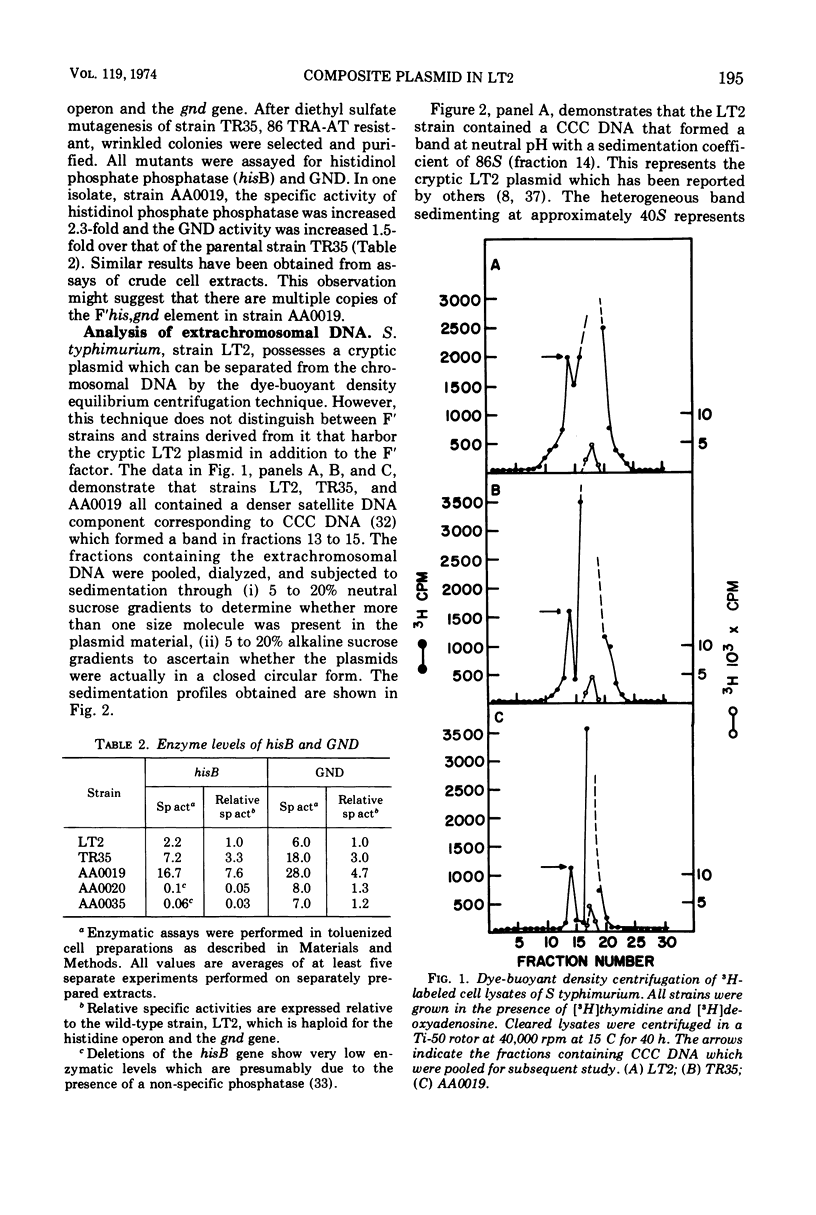
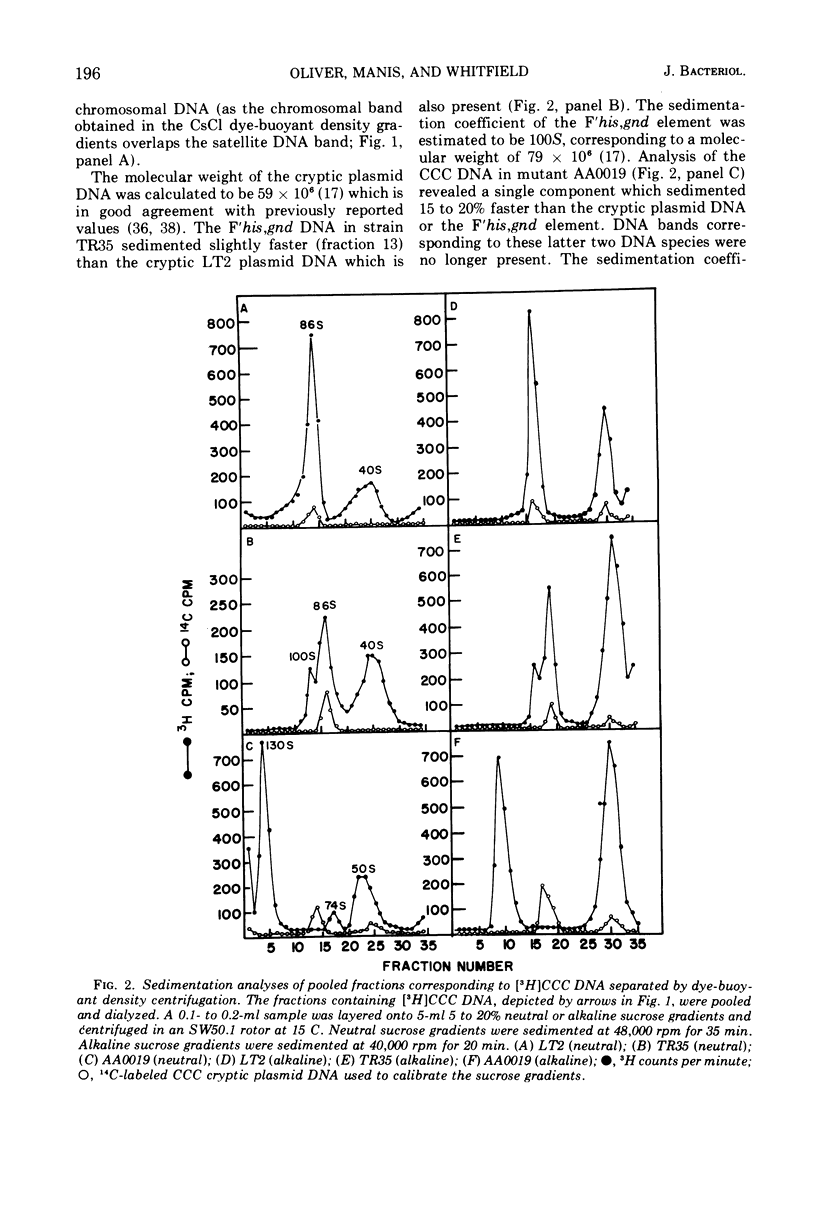
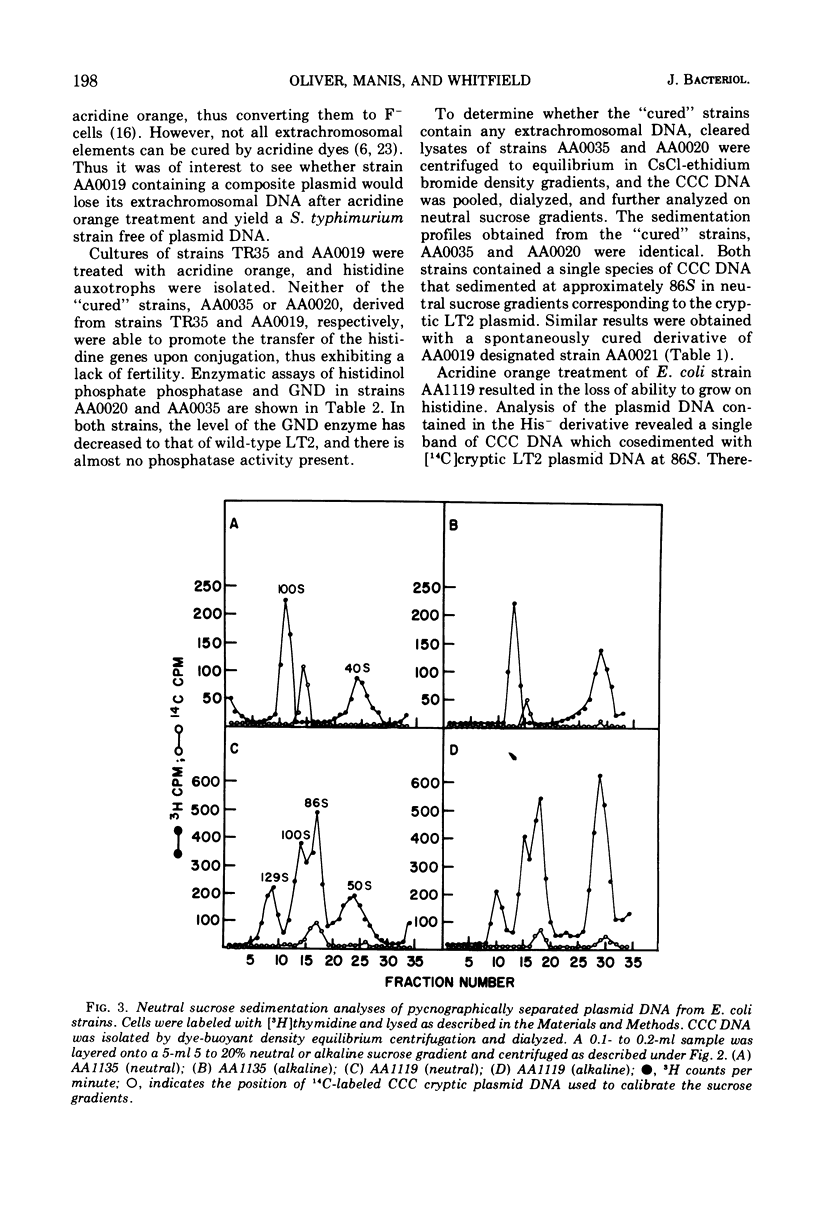
A method designed to select mutants constitutive for expression of the histidine operon has been applied to a Salmonella typhimurium LT2 strain containing an F′his,gnd element and a cryptic plasmid. One of the mutants isolated, strain AA0019, has not only increased levels of histidinol phosphate phosphatase (hisB), but also increased levels of gluconate-6-phosphate dehydrogenase (gnd). Ultracentrifugation studies of extrachromosomal deoxyribonucleic acid (DNA) isolated from strain AA0019 revealed the presence of a single species of covalently closed circular (CCC) DNA that sedimented more rapidly through neutral and alkaline sucrose gradients than any of its possible plasmid precursors. From neutral sucrose gradients, sedimentation coefficients of 130, 100, and 86S were derived, corresponding to the CCC DNA of the large plasmid in strain AA0019, the F′his,gnd element and the cryptic LT2 plasmid, respectively. An Escherichia coli plasmid-free strain that upon mating had received the large 130S plasmid also contained 86S and 100S CCC DNA components. A histidine-requiring derivative of strain AA0019 obtained after acridine orange treatment retained the cryptic plasmid DNA. Apparently, the large plasmid in strain AA0019 consists of the F′his,gnd element and the cryptic LT2 plasmid of the parental strain.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- CLWES R. C., MOODY E. E., PRITCHARD R. H. THE ELIMINATION OF EXTRACHROMOSOMAL ELEMENTS IN THYMINELESS STRAINS OF ESCHERICHIA COLI K12. Genet Res. 1965 Feb;6:147–152. doi: 10.1017/s0016672300004018. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Properties of a supercoiled deoxyribonucleic acid-protein relaxation complex and strand specificity of the relaxation event. Biochemistry. 1970 Oct 27;9(22):4428–4440. doi: 10.1021/bi00824a026. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clewell D. B. Nature of Col E 1 plasmid replication in Escherichia coli in the presence of the chloramphenicol. J Bacteriol. 1972 May;110(2):667–676. doi: 10.1128/jb.110.2.667-676.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clowes R. C. Molecular structure of bacterial plasmids. Bacteriol Rev. 1972 Sep;36(3):361–405. doi: 10.1128/br.36.3.361-405.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta A., Parker C. D., Wohlhieter J. A., Baron L. S. Isolation and characterization of the fertility factor P of Vibrio cholerae. J Bacteriol. 1973 Feb;113(2):763–771. doi: 10.1128/jb.113.2.763-771.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dowman J. E., Meynell G. G. Pleiotropic effects of de-repressed bacterial sex factors on colicinogeny and cell wall structure. Mol Gen Genet. 1970;109(1):57–68. doi: 10.1007/BF00334046. [DOI] [PubMed] [Google Scholar]
- FRAENKEL D. G., HORECKER B. L. PATHWAYS OF D-GLUCOSE METABOLISM IN SALMONELLA TYPHINMURIUM. A STUDY OF A MUTANT LACKING PHOSPHOGLUCOSE ISOMERASE. J Biol Chem. 1964 Sep;239:2765–2771. [PubMed] [Google Scholar]
- Fink G. R., Klopotowski T., Ames B. N. Histidine regulatory mutants in Salmonella typhimurium. IV. A positive selection for polar histidine-requiring mutants from histidine operator constitutive mutants. J Mol Biol. 1967 Nov 28;30(1):81–95. doi: 10.1016/0022-2836(67)90245-8. [DOI] [PubMed] [Google Scholar]
- Fink G. R., Roth J. R. Histidine regulatory mutants in Salmonella typhiumium. VI. Dominance studies. J Mol Biol. 1968 May 14;33(3):547–557. doi: 10.1016/0022-2836(68)90305-7. [DOI] [PubMed] [Google Scholar]
- Hilton J. L., Kearney P. C., Ames B. N. Mode of action of the herbicide, 3-amino-1,2,4-triazole(amitrole): inhibition of an enzyme of histidine biosynthesis. Arch Biochem Biophys. 1965 Dec;112(3):544–547. doi: 10.1016/0003-9861(65)90093-7. [DOI] [PubMed] [Google Scholar]
- Hirota Y. THE EFFECT OF ACRIDINE DYES ON MATING TYPE FACTORS IN ESCHERICHIA COLI. Proc Natl Acad Sci U S A. 1960 Jan;46(1):57–64. doi: 10.1073/pnas.46.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudson B., Clayton D. A., Vinograd J. Complex mitochondrial DNA. Cold Spring Harb Symp Quant Biol. 1968;33:435–442. doi: 10.1101/sqb.1968.033.01.050. [DOI] [PubMed] [Google Scholar]
- Ingram L. C. Deoxyribonucleic acid-deoxyribonucleic acid hybridization of R factors. J Bacteriol. 1973 Sep;115(3):1130–1134. doi: 10.1128/jb.115.3.1130-1134.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kupersztoch Y. M., Helinski D. R. A catenated DNA molecule as an intermediate in the replication of the resistance transfer factor R6K in Escherichia coli. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1451–1459. doi: 10.1016/0006-291x(73)91149-2. [DOI] [PubMed] [Google Scholar]
- LEVIN A. P., HARTMAN P. E. ACTION OF A HISTIDINE ANALOGUE, 1,2,4-TRIAZOLE-3-ALANINE, IN SALMONELLA TYPHIMURIUM. J Bacteriol. 1963 Oct;86:820–828. doi: 10.1128/jb.86.4.820-828.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lew K. K., Roth J. R. Genetic approaches to determination of enzyme quaternary structure. Biochemistry. 1971 Jan 19;10(2):204–207. doi: 10.1021/bi00778a002. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Balbinder E., Bassel A. Molecular characterization of a stable Flac plasmid. Biochem Biophys Res Commun. 1973 Sep 18;54(2):737–743. doi: 10.1016/0006-291x(73)91485-x. [DOI] [PubMed] [Google Scholar]
- Macrina F. L., Balbinder E. Plasmid-associated functions of a stable Flac. J Bacteriol. 1973 Jan;113(1):183–191. doi: 10.1128/jb.113.1.183-191.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray M. L., Hartman P. E. Overproduction of hisH and hisF gene products leads to inhibition of cell cell division in Salmonella. Can J Microbiol. 1972 May;18(5):671–681. doi: 10.1139/m72-105. [DOI] [PubMed] [Google Scholar]
- Murray M. L., Klopotowski T. Genetic map position of the gluconate-6-phosphate dehydrogenase gene in Salmonella typhimurium. J Bacteriol. 1968 Apr;95(4):1279–1282. doi: 10.1128/jb.95.4.1279-1282.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nordström K., Ingram L. C., Lundbäck A. Mutations in R factors of Escherichia coli causing an increased number of R-factor copies per chromosome. J Bacteriol. 1972 May;110(2):562–569. doi: 10.1128/jb.110.2.562-569.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Bouanchaud D. The problems of drug-resistant pathogenic bacteria. Extrachromosomal nature of drug resistance in Staphylococcus aureus. Ann N Y Acad Sci. 1971 Jun 11;182:279–294. doi: 10.1111/j.1749-6632.1971.tb30664.x. [DOI] [PubMed] [Google Scholar]
- Novick R. P. Extrachromosomal inheritance in bacteria. Bacteriol Rev. 1969 Jun;33(2):210–263. doi: 10.1128/br.33.2.210-263.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novick R. P., Smith K., Sheehy R. J., Murphy E. A catenated intermediate in plasmid replication. Biochem Biophys Res Commun. 1973 Oct 15;54(4):1460–1469. doi: 10.1016/0006-291x(73)91150-9. [DOI] [PubMed] [Google Scholar]
- Radloff R., Bauer W., Vinograd J. A dye-buoyant-density method for the detection and isolation of closed circular duplex DNA: the closed circular DNA in HeLa cells. Proc Natl Acad Sci U S A. 1967 May;57(5):1514–1521. doi: 10.1073/pnas.57.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth J. R., Antón D. N., Hartman P. E. Histidine regulatory mutants in Salmonella typhimurium. I. Isolation and general properties. J Mol Biol. 1966 Dec 28;22(2):305–323. doi: 10.1016/0022-2836(66)90134-3. [DOI] [PubMed] [Google Scholar]
- Rownd R., Nakaya R., Nakamura A. Molecular nature of the drug-resistance factors of the Enterobacteriaceae. J Mol Biol. 1966 Jun;17(2):376–393. doi: 10.1016/s0022-2836(66)80149-3. [DOI] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith H. R., Humphreys G. O., Grindley N. D., Grindley J. N., Anderson E. S. Molecular studies of an fi+ plasmid from strains of Salmonella typhimurium. Mol Gen Genet. 1973 Nov 2;126(2):143–151. doi: 10.1007/BF00330989. [DOI] [PubMed] [Google Scholar]
- Spratt B. G. Replication of extrachromosomal elements in a DNA synthesis initiation mutant of Salmonella typhimurium. Biochem Biophys Res Commun. 1972 Aug 7;48(3):496–501. doi: 10.1016/0006-291x(72)90375-0. [DOI] [PubMed] [Google Scholar]
- Spratt B. G., Rowbury R. J. The plasmid of Salmonella typhimurium LT2. Mol Gen Genet. 1973 Mar 19;121(4):347–353. doi: 10.1007/BF00433233. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Vapnek D., Lipman M. B., Rupp W. D. Physical properties and mechanism of transfer of R factors in Escherichia coli. J Bacteriol. 1971 Oct;108(1):508–514. doi: 10.1128/jb.108.1.508-514.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll M. J. Derivation of an F-merogenote and a phi-80 high-frequency transducing phage carrying the histidine operon os Salmonella. J Bacteriol. 1972 Feb;109(2):741–750. doi: 10.1128/jb.109.2.741-750.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willetts N., Bastarrachea F. Genetic and physicochemical characterization of Escherichia coli strains carrying fused F' elements derived from KLF1 and F57. Proc Natl Acad Sci U S A. 1972 Jun;69(6):1481–1485. doi: 10.1073/pnas.69.6.1481. [DOI] [PMC free article] [PubMed] [Google Scholar]


