Abstract
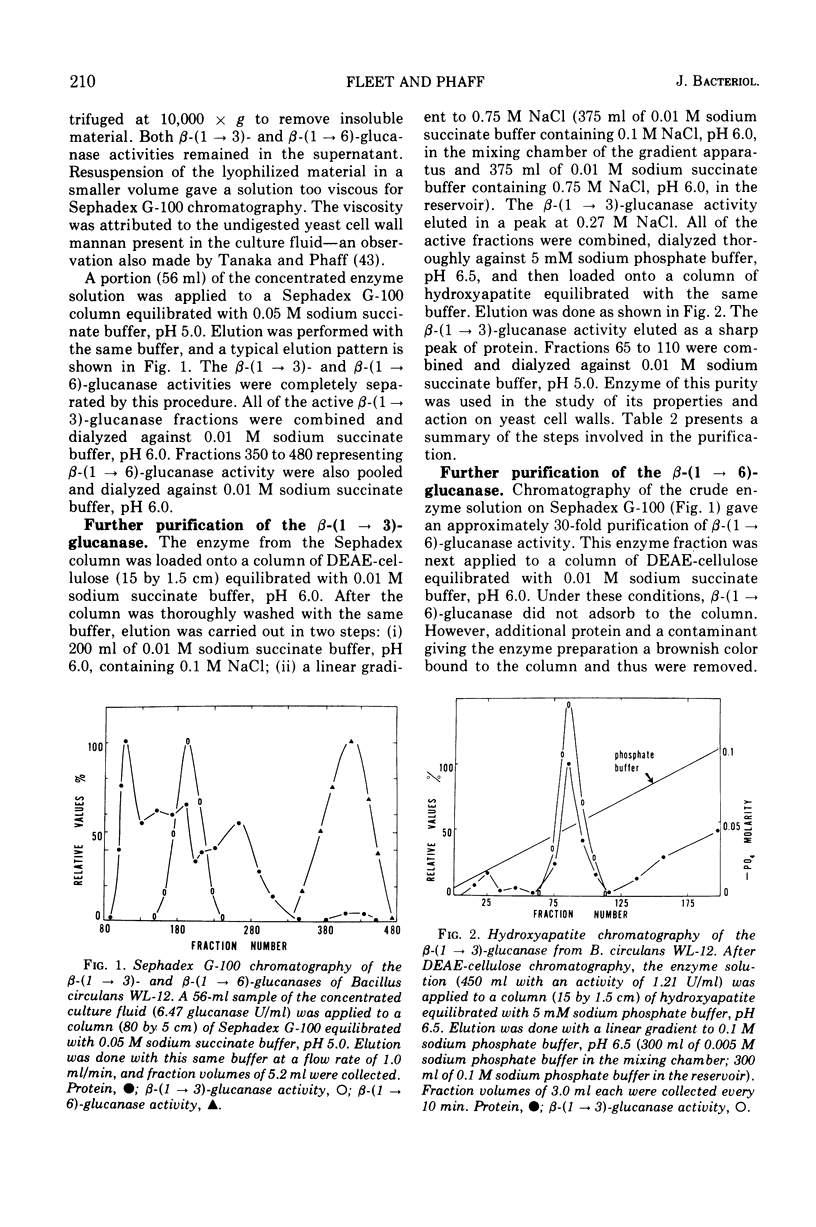
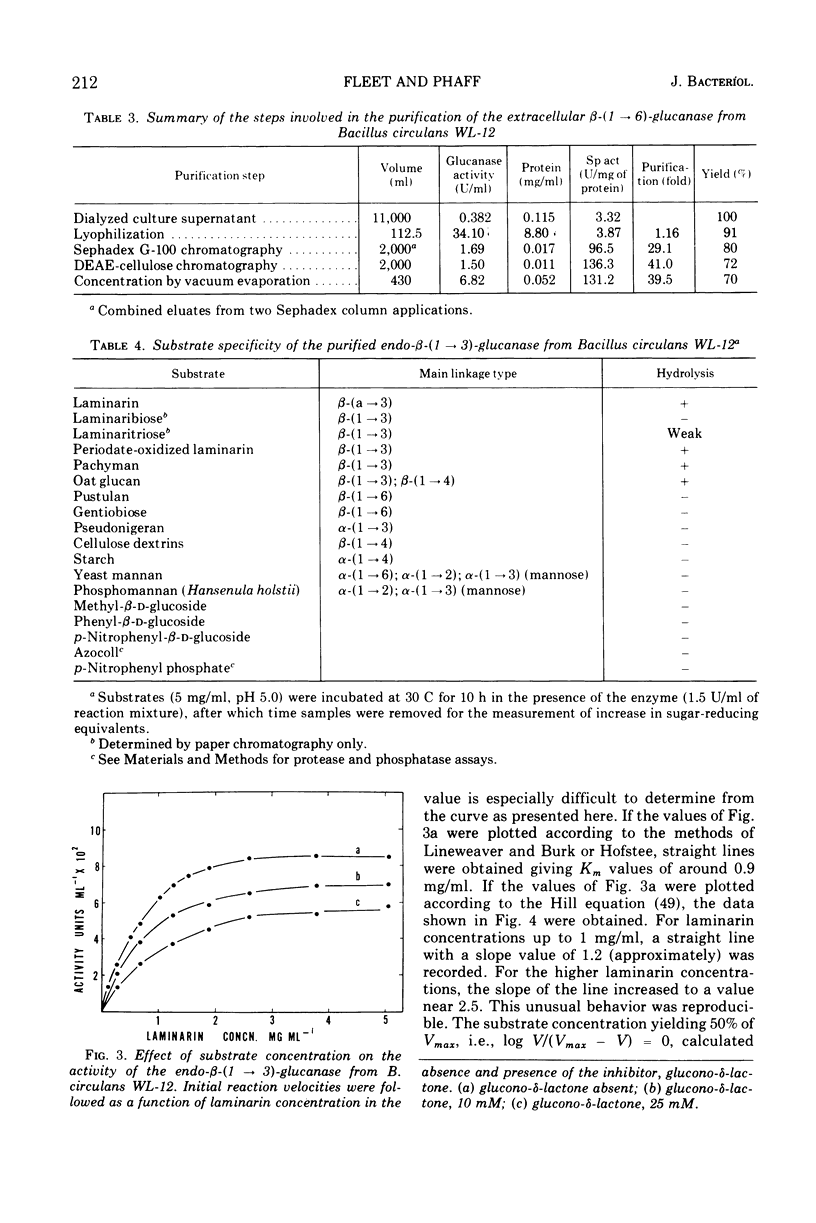
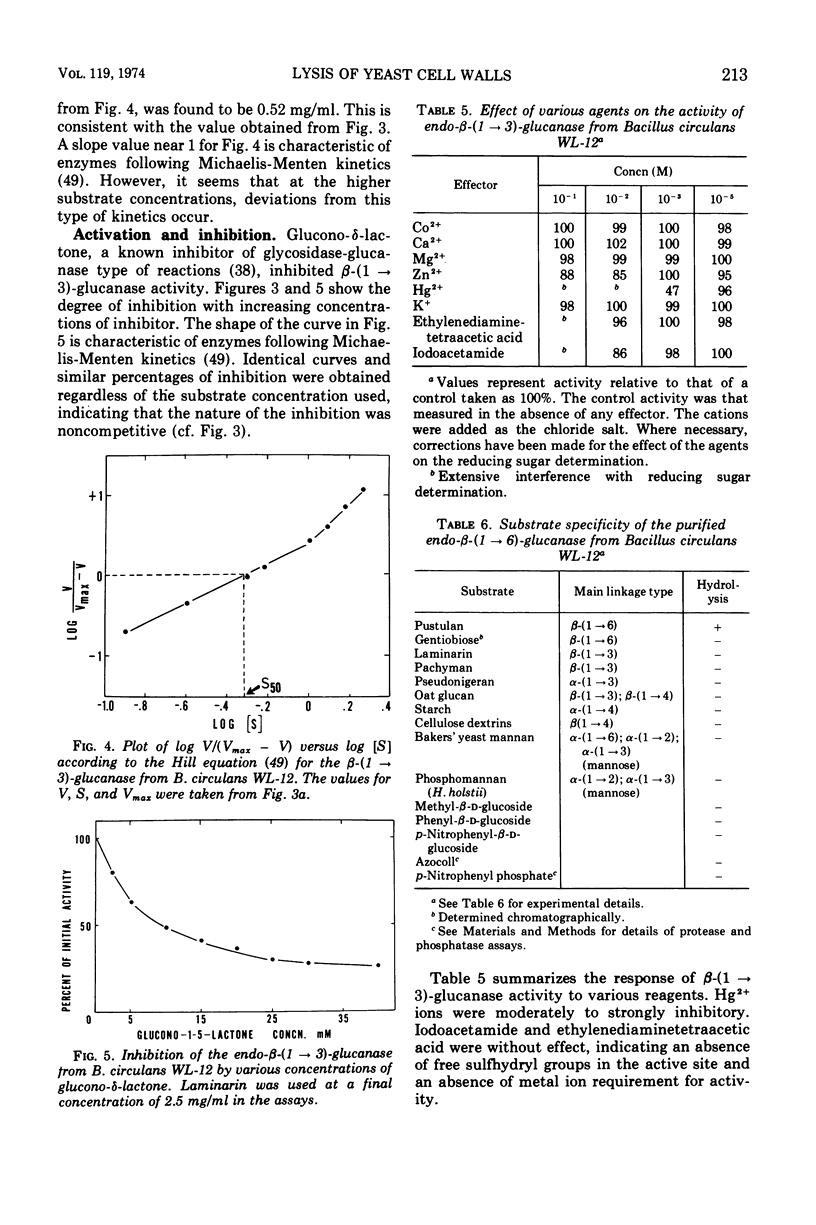
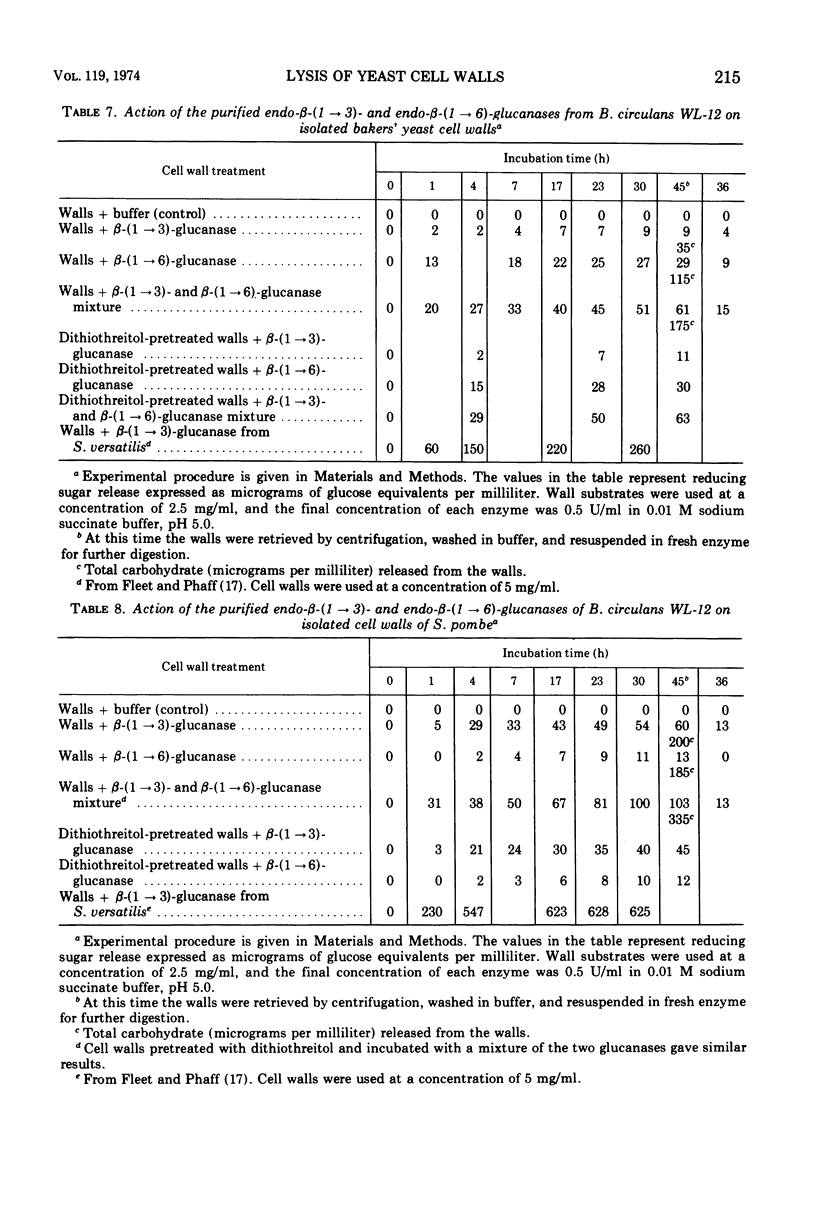
Endo-β-(1 → 3)- and endo-β-(1 → 6)-glucanases are produced in high concentration in the culture fluid of Bacillus circulans WL-12 when grown in a mineral medium with bakers' yeast cell walls as the sole carbon source. Much lower enzyme levels were found when laminarin, pustulan, or mannitol was the substrate. The two enzyme activities were well separated during Sephadex G-100 chromatography. The endo-β-(1 → 3)-glucanase was further purified by diethylaminoethyl-cellulose and hydroxyapatite chromatography, whereas the endo-β-(1 → 6)-glucanase could be purified further by diethylamino-ethyl-cellulose and carboxymethyl cellulose chromatography. The endo-β-(1 → 3)-glucanase was specific for the β-(1 → 3)-glucosidic bond, but it did not hydrolyze laminaribiose; laminaritriose was split very slowly. β-(1 → 4)-Bonds in oat glucan in which the glucosyl moiety is substituted in the 3-position were also cleaved. The kinetics of laminarin hydrolysis (optimum pH 5.0) were complex but appeared to follow Michaelis-Menten theory, especially at the lower substrate concentrations. Glucono-δ-lactone was a noncompetitive inhibitor and Hg2+ inhibited strongly. The enzyme has no metal ion requirements or essential sulfhydryl groups. The purified β-(1 → 6)-glucanase has an optimum pH of 5.5, and its properties were studied in less detail. In contrast to the crude culture fluid, the two purified β-glucanases have only a very limited hydrolytic action on cell wall of either bakers' yeast or of Schizosaccharomyces pombe. Although our previous work had assumed that the two glucanases studied here are responsible for cell wall lysis, it now appears that the culture fluid contains in addition a specific lytic enzyme which is eliminated during the extensive purification process.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abd-el-Al A. T., Phaff H. J. Exo-beta-glucanases in yeast. Biochem J. 1968 Sep;109(3):347–360. doi: 10.1042/bj1090347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abd-el-Al A. T., Phaff H. J. Purification and properties of endo-beta-glucanase in the yeast Hanseniaspora valbyensis. Can J Microbiol. 1969 Jul;15(7):697–701. doi: 10.1139/m69-123. [DOI] [PubMed] [Google Scholar]
- Abeles F. B., Bosshart R. P., Forrence L. E., Habig W. H. Preparation and purification of glucanase and chitinase from bean leaves. Plant Physiol. 1971 Jan;47(1):129–134. doi: 10.1104/pp.47.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnold W. N. The structure of the yeast cell wall. Solubilization of a marker enzyme, -fructofuranosidase, by the autolytic enzyme system. J Biol Chem. 1972 Feb 25;247(4):1161–1169. [PubMed] [Google Scholar]
- Bacon J. S., Gordon A. H., Jones D., Taylor I. F., Webley D. M. The separation of beta-glucanases produced by Cytophaga johnsonii and their role in the lysis of yeast cell walls. Biochem J. 1970 Nov;120(1):67–78. doi: 10.1042/bj1200067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon J. S., Gordon A. H., Webley D. M. Fractionation of the beta-glucanases in a Cytophage johnsonii culture filtrate lysing yeast cell walls. Biochem J. 1970 Apr;117(2):42P–43P. doi: 10.1042/bj1170042pb. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull A. T., Chesters C. G. The biochemistry of laminarin and the nature of laminarinase. Adv Enzymol Relat Areas Mol Biol. 1966;28:325–364. doi: 10.1002/9780470122730.ch5. [DOI] [PubMed] [Google Scholar]
- Cabib E., Ulane R. Chitin synthetase activating factor from yeast, a protease. Biochem Biophys Res Commun. 1973 Jan 4;50(1):186–191. doi: 10.1016/0006-291x(73)91081-4. [DOI] [PubMed] [Google Scholar]
- Doi K., Doi A., Fukui T. Joint action of two glucanases produced by Arthrobacter in spheroplast formation from baker's yeast. J Biochem. 1971 Oct;70(4):711–714. doi: 10.1093/oxfordjournals.jbchem.a129686. [DOI] [PubMed] [Google Scholar]
- Doi K., Doi A., Ozaki T., Fukui T. Heterogeneity of the lytic activity for yeast cell wall observed among the components of an Arthrobacter glucanase. J Biochem. 1973 Mar;73(3):667–670. doi: 10.1093/oxfordjournals.jbchem.a130127. [DOI] [PubMed] [Google Scholar]
- Fleet G. H., Phaff H. J. Glucanases in Schizosaccharomyces. Isolation and properties of the cell wall-associated beta(1 leads to 3)-glucanases. J Biol Chem. 1974 Mar 25;249(6):1717–1728. [PubMed] [Google Scholar]
- HJERTEN S., LEVIN O., TISELIUS A. Protein chromatography on calcium phosphate columns. Arch Biochem Biophys. 1956 Nov;65(1):132–155. doi: 10.1016/0003-9861(56)90183-7. [DOI] [PubMed] [Google Scholar]
- HORIKOSHI K., KOFFLER H., ARIMA K. Purification and properties of beta-1,3-glucanase from the "lytic enzyme" of Bacillus circulans. Biochim Biophys Acta. 1963 Jun 11;73:267–275. doi: 10.1016/0006-3002(63)90311-1. [DOI] [PubMed] [Google Scholar]
- Hasegawa S., Nordin J. H. Enzymes that hydrolyze fungal cell wall polysaccharides. I. Purification and properties of an endo-alpha-D-(1-3)-glucanase from Trichoderma. J Biol Chem. 1969 Oct 25;244(20):5460–5470. [PubMed] [Google Scholar]
- Hoffmann G. C., Simson B. W., Timell T. E. Structure and molecular size of pachyman. Carbohydr Res. 1971 Nov;20(1):185–188. doi: 10.1016/s0008-6215(00)84962-9. [DOI] [PubMed] [Google Scholar]
- Johnston I. R. The partial acid hydrolysis of a highly dextrorotatory fragment of the cell wall of Aspergillus niger. Isolation of the alpha-(1-3)-linked dextrin series. Biochem J. 1965 Sep;96(3):659–664. doi: 10.1042/bj0960659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura K., Yamamoto Y. Purification and properties of an enzyme, zymolyase, which lyses viable yeast cells. Arch Biochem Biophys. 1972 Nov;153(1):403–406. doi: 10.1016/0003-9861(72)90461-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mann J. W., Heintz C. E., Macmillan J. D. Yeast spheroplasts formed by cell wall-degrading enzymes from Oerskovia sp. J Bacteriol. 1972 Sep;111(3):821–824. doi: 10.1128/jb.111.3.821-824.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners D. J., Masson A. J., Patterson J. C., Björndal H., Lindberg B. The structure of a beta-(1--6)-D-glucan from yeast cell walls. Biochem J. 1973 Sep;135(1):31–36. doi: 10.1042/bj1350031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manners D. J., Masson A. J., Patterson J. C. The structure of a beta-(1 leads to 3)-D-glucan from yeast cell walls. Biochem J. 1973 Sep;135(1):19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan W. L., Jr, Lampen J. O. Phosphomannanase (PR-factor), an enzyme required for the formation of yeast protoplasts. J Bacteriol. 1968 Mar;95(3):967–974. doi: 10.1128/jb.95.3.967-974.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLellan W. L., Jr, McDaniel L. E., Lampen J. O. Purification of phosphomannanase and its action on the yeast cell wall. J Bacteriol. 1970 Apr;102(1):261–270. doi: 10.1128/jb.102.1.261-270.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore A. E., Stone B. A. A -I,3-glucan hydrolase from Nicotiana glutinosa. I. Extraction, purification and physical properties. Biochim Biophys Acta. 1972 Jan 20;258(1):238–247. doi: 10.1016/0005-2744(72)90982-5. [DOI] [PubMed] [Google Scholar]
- Moore A. E., Stone B. A. A -I,3-glucan hydrolase from Nicotiana glutinosa. II. Specificity, action pattern and inhibitor studies. Biochim Biophys Acta. 1972 Jan 20;258(1):248–264. doi: 10.1016/0005-2744(72)90983-7. [DOI] [PubMed] [Google Scholar]
- REESE E. T., MANDELS M. Beta-D-1, 3 Glucanases in fungi. Can J Microbiol. 1959 Apr;5(2):173–185. doi: 10.1139/m59-022. [DOI] [PubMed] [Google Scholar]
- REESE E. T., MANDELS M. Use of enzymes in isolation and analysis of polysaccharides. Appl Microbiol. 1959 Nov;7:378–387. doi: 10.1128/am.7.6.378-387.1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REESE E. T., PARRISH F. W., MANDELS M. Beta-d-1, 6-Glucanases in fungi. Can J Microbiol. 1962 Jun;8:327–334. doi: 10.1139/m62-045. [DOI] [PubMed] [Google Scholar]
- TANAKA H., PHAFF H. J. ENZYMATIC HYDROLYSIS OF YEAST CELL WALLS. I. ISOLATION OF WALL-DECOMPOSING ORGANISMS AND SEPARATION AND PURIFICATION OF LYTIC ENZYMES. J Bacteriol. 1965 Jun;89:1570–1580. doi: 10.1128/jb.89.6.1570-1580.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Yamamoto S., Shiraishi T., Nagasaki S. Crystalline enzyme which degrades the cell wall of living yeast. Biochem Biophys Res Commun. 1972 Mar 10;46(5):1802–1809. doi: 10.1016/0006-291x(72)90054-x. [DOI] [PubMed] [Google Scholar]


