Abstract
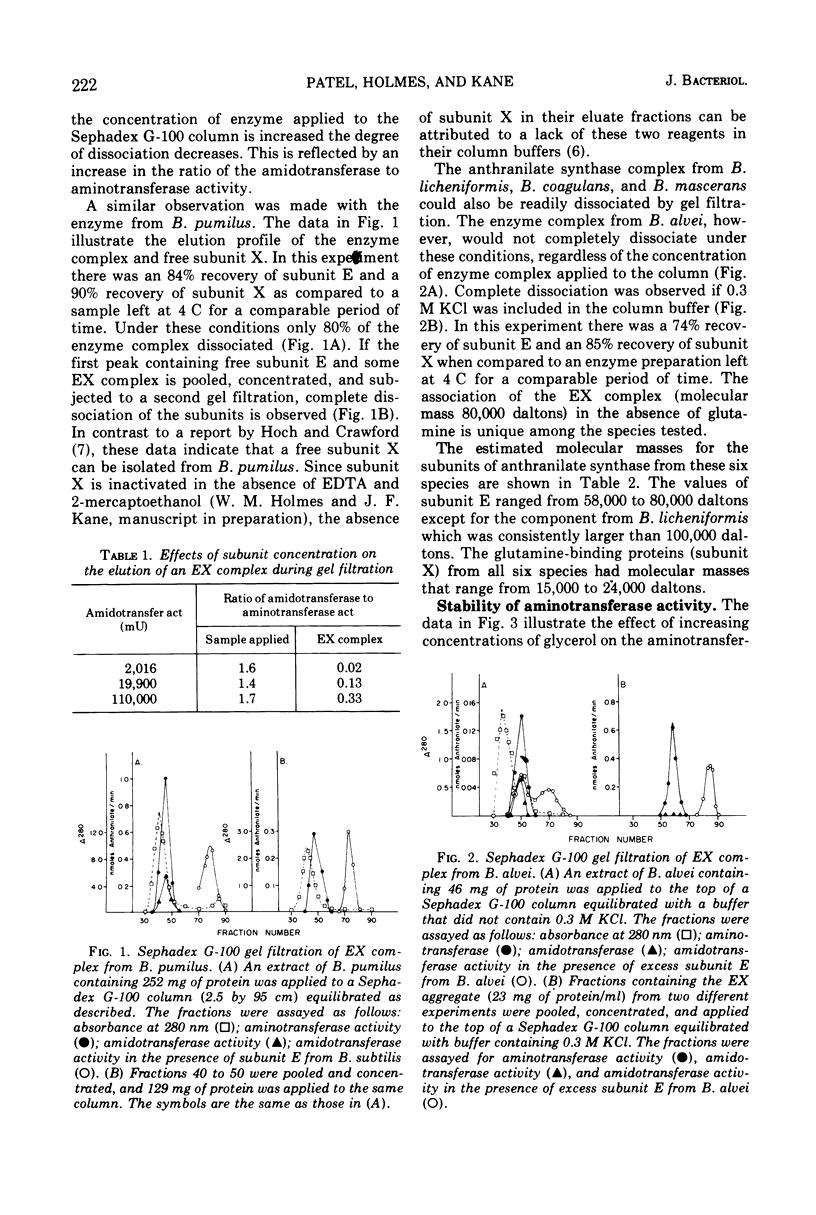
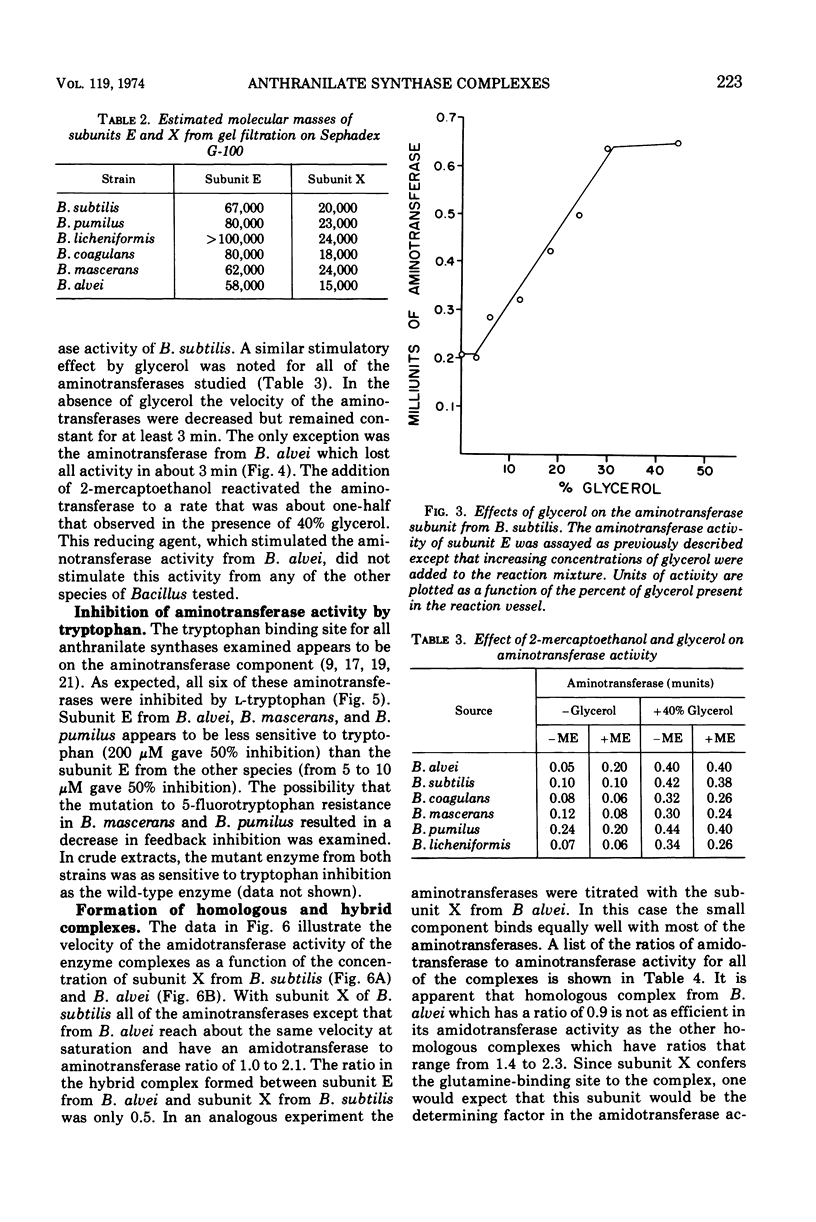
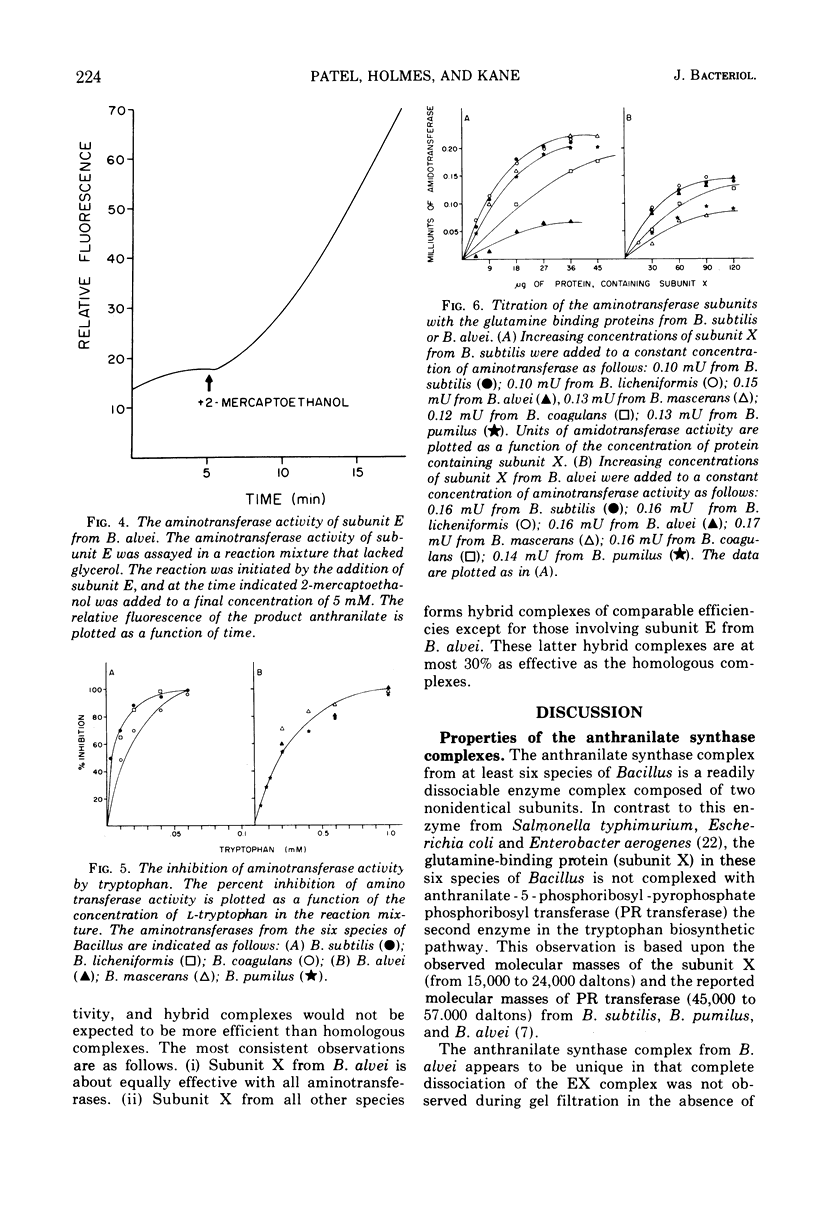
The subunits of anthranilate synthase were separated and partially purified by Sephadex G-100 gel filtration from the following six species of Bacillus: Bacillus subtilis, Bacillus licheniformis, Bacillus alvei, Bacillus coagulans, Bacillus pumilus, and Bacillus mascerans. Our data suggest that the enzyme from B. alvei is unique among these species. First, the anthranilate synthase complexes are readily dissociated during gel filtration in the absence of glutamine into a large component (aminotransferase), subunit E, and a small component subunit X (glutamine-binding protein), whereas a higher salt concentration is required to dissociate the complex from B. alvei. Second, the aminotransferase activity from all six species is stimulated by glycerol and inhibited by tryptophan; however, only the large component from B. alvei is stimulated by 2-mercaptoethanol. Finally, the large component can be titrated with the small component to yield a complex which can utilize glutamine as a substrate (amidotransferase). The homologous complexes have an amidotransferase to aminotransferase ratio of 1.4 to 2.3, but the B. alvei complex has a ratio of 0.9. Except for complexes that involve the large component from B. alvei, hybrid complexes can be formed which have ratios as good as the homologous complexes. These data are consistent with the hypothesis that B. alvei is unique among the bacilli with respect to some enzymes in the aromatic amino acid biosynthetic pathway.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baskerville E. N., Twarog R. Regulation of the tryptophan synthetic enzymes in Clostridium butyricum. J Bacteriol. 1972 Oct;112(1):304–314. doi: 10.1128/jb.112.1.304-314.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catena A., DeMoss R. D. Physiological and kinetic studies with anthranilate synthetase of Bacillus alvei. J Bacteriol. 1970 Feb;101(2):476–482. doi: 10.1128/jb.101.2.476-482.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch J. A., Demoss R. D. Physiological Effects of a Constitutive Tryptophanase in Bacillus alvei. J Bacteriol. 1965 Sep;90(3):604–610. doi: 10.1128/jb.90.3.604-610.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S. O., Anagnostopoulos C., Crawford I. P. Enzymes of the tryptophan operon of Bacillus subtilis. Biochem Biophys Res Commun. 1969 Jun 27;35(6):838–844. doi: 10.1016/0006-291x(69)90700-1. [DOI] [PubMed] [Google Scholar]
- Hoch S. O., Crawford I. P. Enzymes of the tryptophan pathway in three Bacillus species. J Bacteriol. 1973 Nov;116(2):685–693. doi: 10.1128/jb.116.2.685-693.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch S. O. Tryptophan synthetase from Bacillus subtilis. Purification and characterization of the component. J Biol Chem. 1973 May 10;248(9):2999–3003. [PubMed] [Google Scholar]
- Ito J. Hybrid anthranilate synthetase molecules from enterobacterial sources. Nature. 1969 Jul 5;223(5201):57–59. doi: 10.1038/223057a0. [DOI] [PubMed] [Google Scholar]
- Ito J., Yanofsky C. Anthranilate synthetase, an enzyme specified by the tryptophan operon of Escherichia coli: Comparative studies on the complex and the subunits. J Bacteriol. 1969 Feb;97(2):734–742. doi: 10.1128/jb.97.2.734-742.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A. A biochemical basis for apparent abortive transformation in Bacillus subtilis. Genetics. 1968 Dec;60(4):707–717. doi: 10.1093/genetics/60.4.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen R. A., Nasser D. S., Nester E. W. Comparative control of a branch-point enzyme in microorganisms. J Bacteriol. 1967 Nov;94(5):1582–1593. doi: 10.1128/jb.94.5.1582-1593.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane J. F., Holmes W. M., Jensen R. A. Metabolic interlock. The dual function of a folate pathway gene as an extra-operonic gene of tryptophan biosynthesis. J Biol Chem. 1972 Mar 10;247(5):1587–1596. [PubMed] [Google Scholar]
- Kane J. F., Jensen R. A. Metabolic interlock. The influence of histidine on tryptophan biosynthesis in Bacillus subtilis. J Biol Chem. 1970 May 10;245(9):2384–2390. [PubMed] [Google Scholar]
- Kane J. F., Jensen R. A. The molecular aggregation of anthranilate synthase in Bacillus subtilis. Biochem Biophys Res Commun. 1970 Oct 23;41(2):328–333. doi: 10.1016/0006-291x(70)90507-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Patel N., Holmes W. M., Kane J. F. Intergeneric complementation of anthranilate synthase subunits. J Bacteriol. 1973 May;114(2):600–602. doi: 10.1128/jb.114.2.600-602.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Queener S. F., Gunsalus I. C. Anthranilate synthase enzyme system and complementation in Pseudomonas species. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1225–1232. doi: 10.1073/pnas.67.3.1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb F., Belser W. L. Anthranilate synthetase. In vitro complementation between Serratia marcescens and Escherichia coli subunits. Biochim Biophys Acta. 1972 Nov 28;285(1):243–252. [PubMed] [Google Scholar]
- Sawula R. V., Crawford I. P. Anthranilate synthetase of Acinetobacter calcoaceticus. Separation and partial characterization of subunits. J Biol Chem. 1973 May 25;248(10):3573–3581. [PubMed] [Google Scholar]
- Zalkin H. Anthranilate synthetase. Adv Enzymol Relat Areas Mol Biol. 1973;38:1–39. doi: 10.1002/9780470122839.ch1. [DOI] [PubMed] [Google Scholar]
- Zalkin H., Kling D. Anthranilate synthetase. Purification and properties of component I from Salmonella typhimurium. Biochemistry. 1968 Oct;7(10):3566–3573. doi: 10.1021/bi00850a034. [DOI] [PubMed] [Google Scholar]


