Abstract
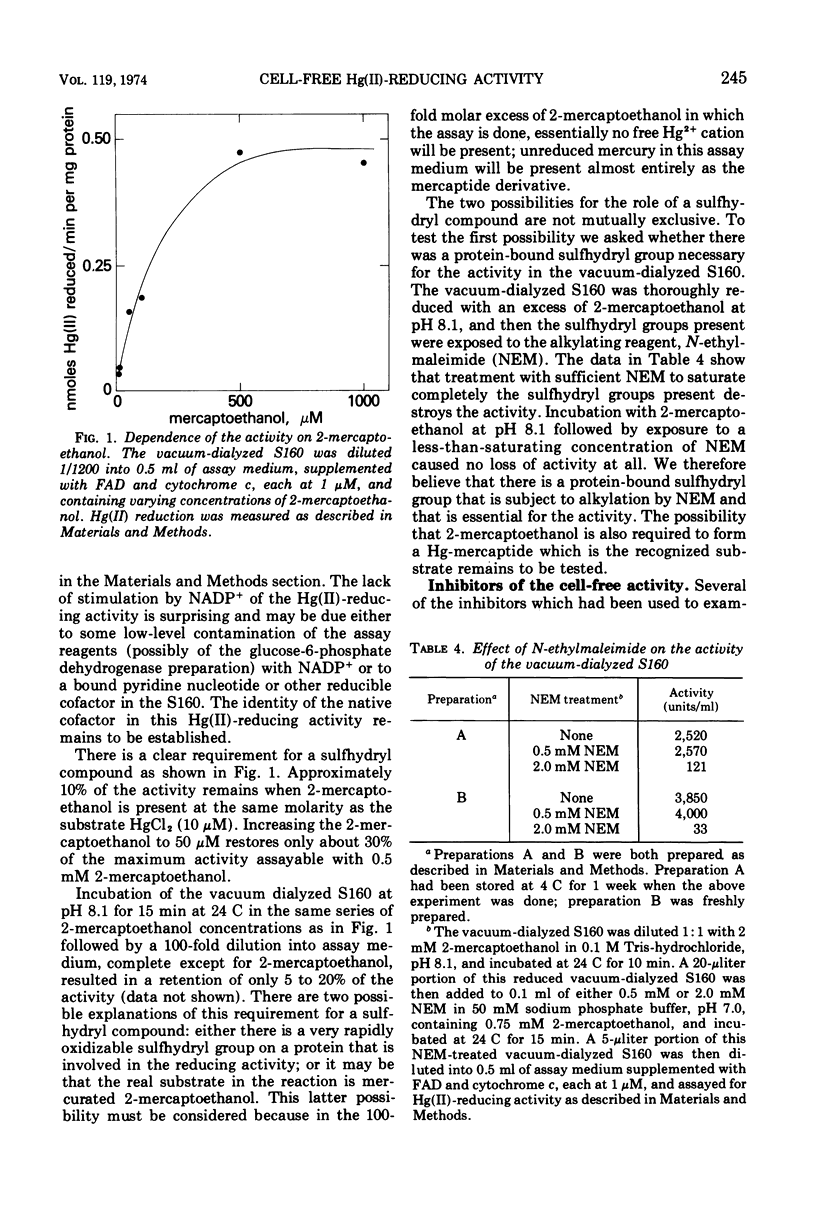
The ability to reduce Hg(II) to Hg(0), which is determined by a plasmid-borne gene in Escherichia coli, is conferred by a Hg(II)-inducible activity which is located in the cytoplasm rather than in the periplasmic space of the cell. This Hg(II)-reducing activity can be isolated from the supernatant of a 160,000 × g centrifugation after French Press disruption of the cells. The activity is dependent on glucose-6-phosphate, glucose-6-phosphate dehydrogenase, and 2-mercaptoethanol, but is not enhanced by added nicotinamide adenine dinucleotide phosphate. Treatment of the active fraction with N-ethylmaleimide causes irreversible loss of the Hg(II)-reducing activity. Unlike the Hg(II)-reducing activity found in intact cells, the cell-free activity is not inhibited by toluene, potassium cyanide, or m-chlorocarbonylcyanide-phenylhydrazone; however, it is inhibited by Ag(I) and phenylmercuric acetate to the same extent as the activity in intact cells. Neither phenylmercuric acetate nor methylmercuric chloride is reduced to Hg(0) by the cell-free activity. Au(III), however, is a substrate for the cell-free activity; it is reduced to metallic colloidal Au(0).
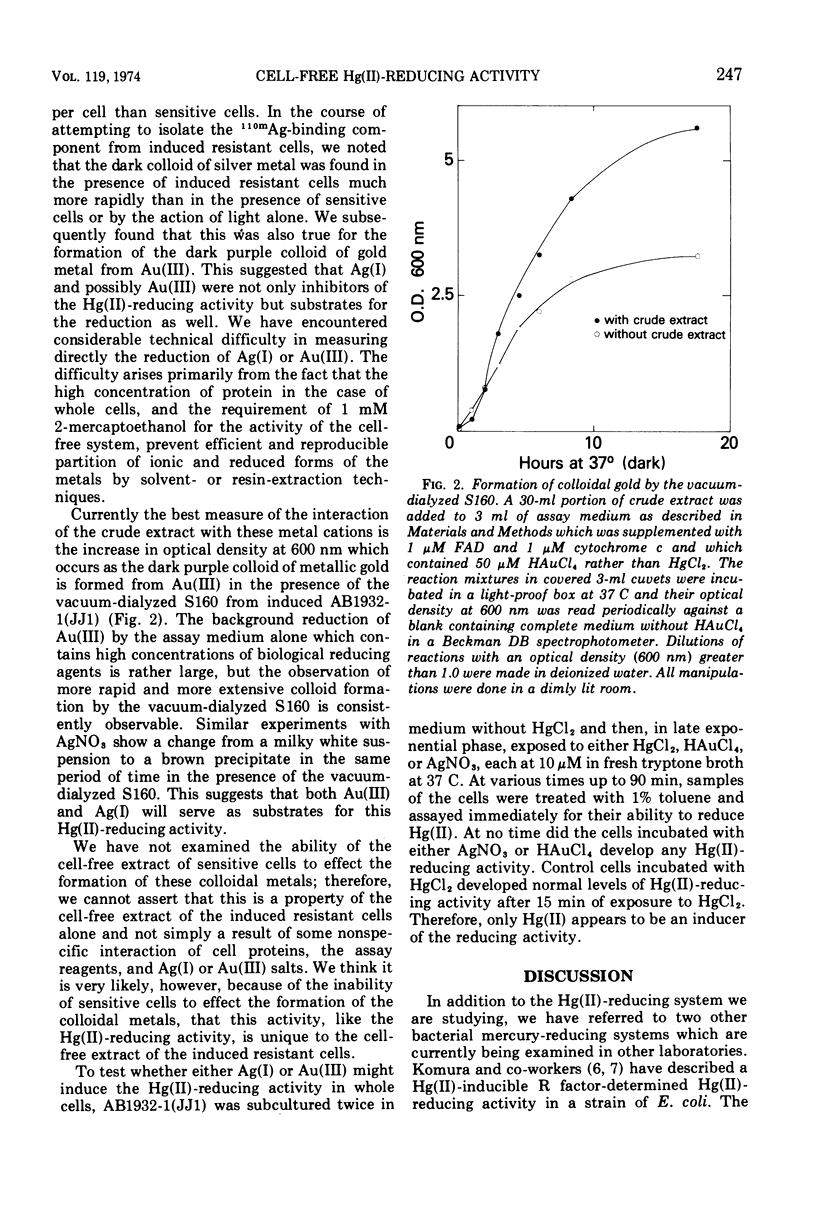
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Harwood J. H., Smith D. H. Resistance factor-mediated streptomycin resistance. J Bacteriol. 1969 Mar;97(3):1262–1271. doi: 10.1128/jb.97.3.1262-1271.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harwood J., Smith D. H. Catabolite repression of chloramphenicol acetyl transferase synthesis in E. coli K12. Biochem Biophys Res Commun. 1971 Jan 8;42(1):57–62. doi: 10.1016/0006-291x(71)90361-5. [DOI] [PubMed] [Google Scholar]
- Komura I., Funaba T., Izaki K. Mechanism of mercuric chloride resistance in microorganisms. II. NADPH-dependent reduction of mercuric chloride and vaporization of mercury from mercuric chloride by a multiple drug resistant strain of Escherichia coli. J Biochem. 1971 Dec;70(6):895–901. doi: 10.1093/oxfordjournals.jbchem.a129719. [DOI] [PubMed] [Google Scholar]
- Komura I., Izaki K. Mechanism of mercuric chloride resistance in microorganisms. I. Vaporization of a mercury compound from mercuric chloride by multiple drug resistant strains of Escherichia coli. J Biochem. 1971 Dec;70(6):885–893. doi: 10.1093/oxfordjournals.jbchem.a129718. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MAGOS L., TUFFERY A. A., CLARKSON T. W. VOLATILIZATION OF MERCURY BY BACTERIA. Br J Ind Med. 1964 Oct;21:294–298. doi: 10.1136/oem.21.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu H. C., Winshell E. B. Purification and characterization of penicillinases from Salmonella typhimurium and Escherichia coli. Arch Biochem Biophys. 1970 Aug;139(2):278–290. doi: 10.1016/0003-9861(70)90479-0. [DOI] [PubMed] [Google Scholar]
- Nossal N. G., Heppel L. A. The release of enzymes by osmotic shock from Escherichia coli in exponential phase. J Biol Chem. 1966 Jul 10;241(13):3055–3062. [PubMed] [Google Scholar]
- Shaw W. V. The problems of drug-resistant pathogenic bacteria. Comparative enzymology of chloramphenicol resistance. Ann N Y Acad Sci. 1971 Jun 11;182:234–242. doi: 10.1111/j.1749-6632.1971.tb30660.x. [DOI] [PubMed] [Google Scholar]
- Summers A. O., Lewis E. Volatilization of mercuric chloride by mercury-resistant plasmid-bearing strains of Escherichia coli, Staphylococcus aureus, and Pseudomonas aeruginosa. J Bacteriol. 1973 Feb;113(2):1070–1072. doi: 10.1128/jb.113.2.1070-1072.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summers A. O., Silver S. Mercury resistance in a plasmid-bearing strain of Escherichia coli. J Bacteriol. 1972 Dec;112(3):1228–1236. doi: 10.1128/jb.112.3.1228-1236.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood J. M., Kennedy F. S., Rosen C. G. Synthesis of methyl-mercury compounds by extracts of a methanogenic bacterium. Nature. 1968 Oct 12;220(5163):173–174. doi: 10.1038/220173a0. [DOI] [PubMed] [Google Scholar]



