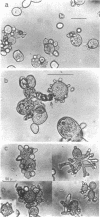
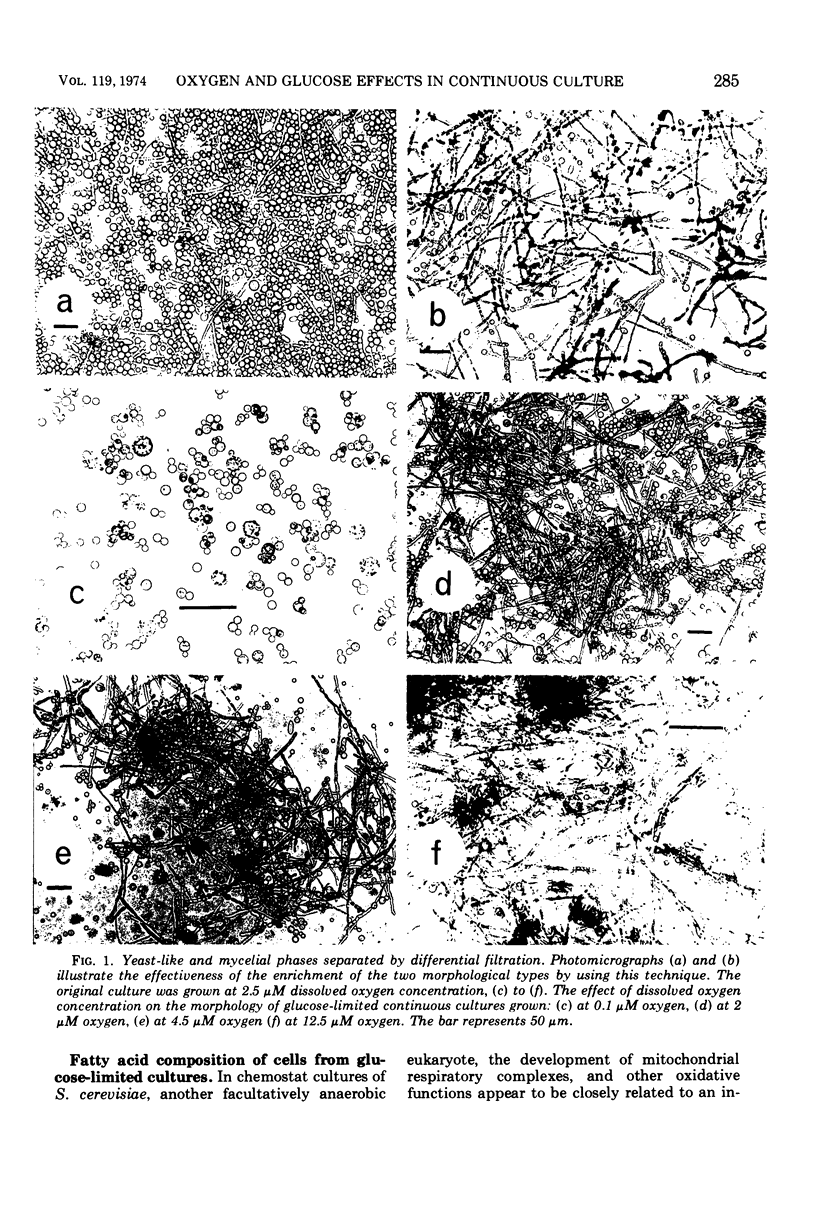
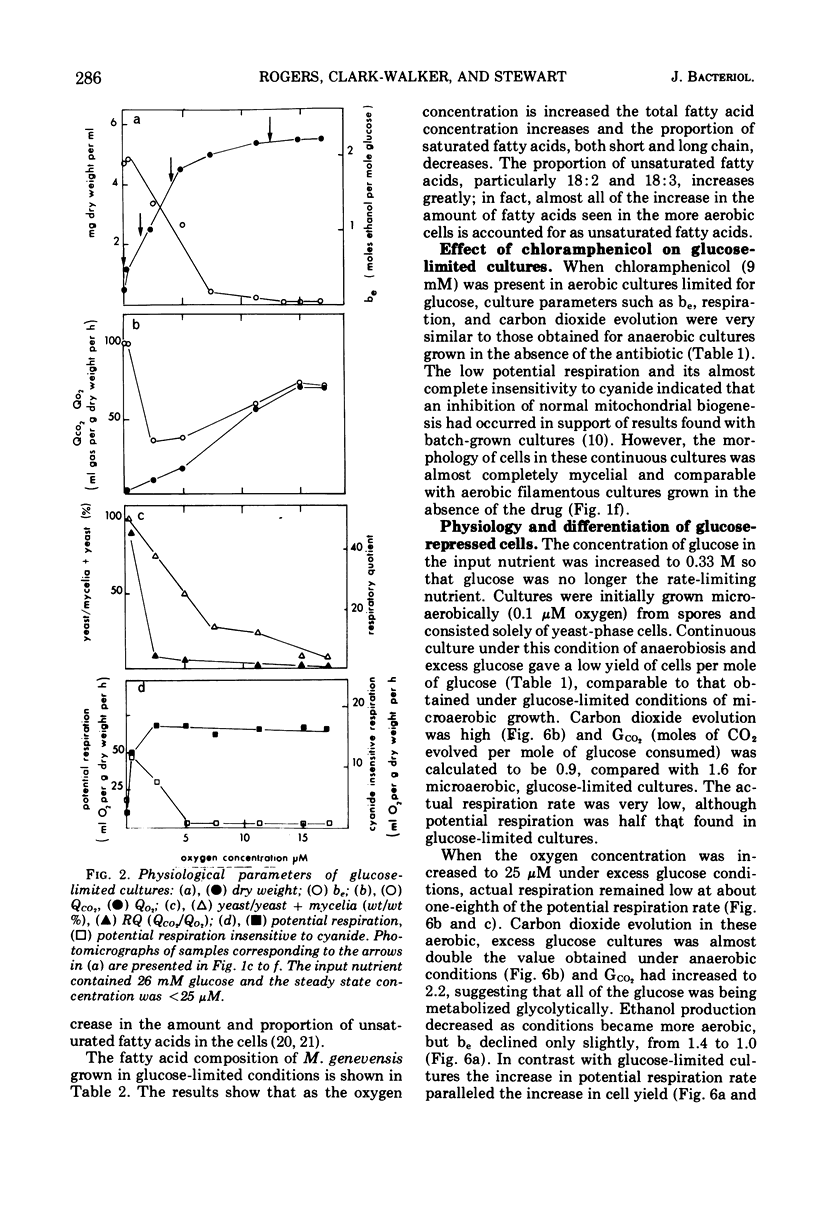
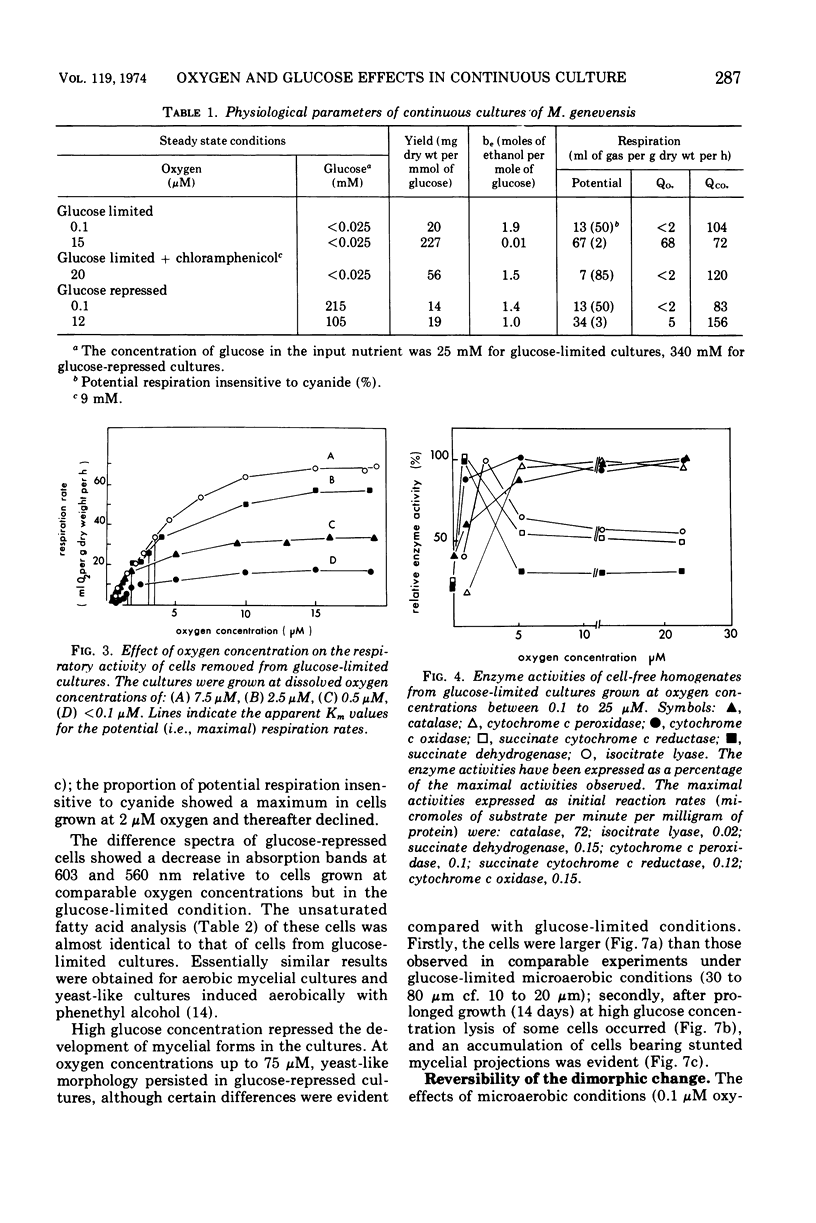
Abstract
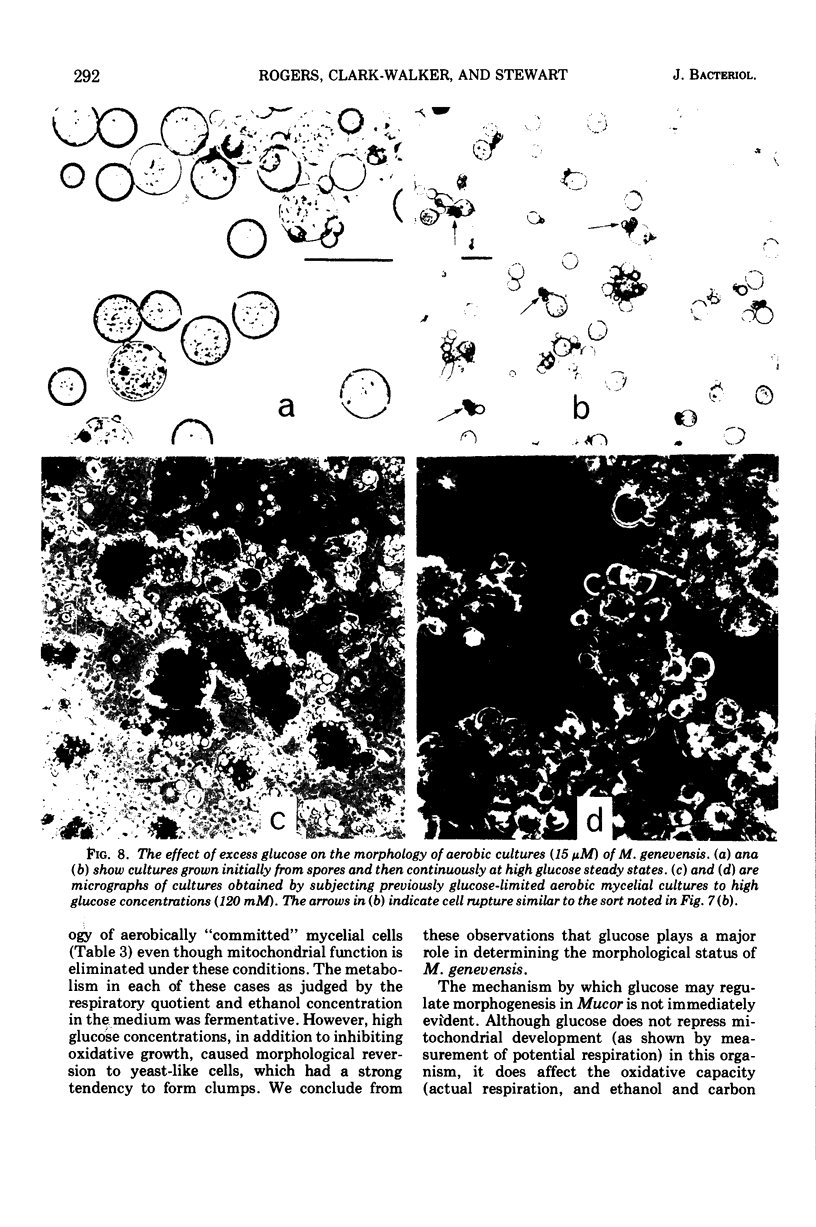
Mucor genevensis was grown in both glucose-limited and glucose-excess continuous cultures over a range of dissolved oxygen concentrations (<0.1 to 25 μM) to determine the effects of glucose and the influence of metabolic mode (fermentative versus oxidative) on dimorphic transformations in this organism. The extent of differentiation between yeast and mycelial phases has been correlated with physiological and biochemical parameters of the cultures. Under glucose limitation, oxidative metabolism increased as the dissolved oxygen concentration increased, and this paralleled the increase in the proportion of the mycelial phase in the cultures. Filamentous growth and oxidative metabolism were both inhibited by glucose even though mitochondrial development was only slightly repressed. However, the presence of chloramphenicol in glucose-limited aerobic cultures inhibited mitochondrial respiratory development but did not induce yeast-like growth, indicating that oxidative metabolism is not essential for mycelial development. Once mycelial cultures had been established under aerobic, glucose-limited conditions, subsequent reversal to anaerobic conditions or treatment with chloramphenicol caused only a limited reversal (<35%) to the yeast-like form. Glucose, however, induced a complete reversion to yeast-like form. It is concluded that glucose is the most important single culture factor determining the morphological status of M. genevensis; mitochondrial development and the functional oxidative capacities of the cell appear to be less important factors in the differentiation process.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTNICKI GARCIA S. SYMPOSIUM ON BIOCHEMICAL BASES OF MORPHOGENESIS IN FUNGI. III. MOLD-YEAST DIMORPHISM OF MUCOR. Bacteriol Rev. 1963 Sep;27:293–304. doi: 10.1128/br.27.3.293-304.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTNICKI-GARCIA S., NICKERSON W. J. Induction of yeast-like development in Mucor by carbon dioxide. J Bacteriol. 1962 Oct;84:829–840. doi: 10.1128/jb.84.4.829-840.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Nickerson W. J. THIAMINE AND NICOTINIC ACID: ANAEROBIC GROWTH FACTORS FOR MUCOR ROUXII. J Bacteriol. 1961 Jul;82(1):142–148. doi: 10.1128/jb.82.1.142-148.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody S. Correlation between reduced nicotinamide adenine dinucleotide phosphate levels and morphological changes in Neurospora crassa. J Bacteriol. 1970 Mar;101(3):802–807. doi: 10.1128/jb.101.3.802-807.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D. Development of respiration and mitochondria in Mucor genevensis after anaerobic growth: absence of glucose repression. J Bacteriol. 1972 Jan;109(1):399–408. doi: 10.1128/jb.109.1.399-408.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark-Walker G. D. Relationship between dimorphology and respiration in Mucor genevensis studied with chloramphenicol. J Bacteriol. 1973 Nov;116(2):972–980. doi: 10.1128/jb.116.2.972-980.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmer G. W., Nickerson W. J. Nutritional requirements for growth and yeastlike development of Mucor rouxii under carbon dioxide. J Bacteriol. 1970 Feb;101(2):595–602. doi: 10.1128/jb.101.2.595-602.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feierabend J., Beevers H. Developmental studies on microbodies in wheat leaves : I. Conditions influencing enzyme development. Plant Physiol. 1972 Jan;49(1):28–32. doi: 10.1104/pp.49.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon P. A., Stewart P. R., Clark-Walker G. D. Fatty acid and sterol composition of Mucor genevensis in relation to dimorphism and anaerobic growth. J Bacteriol. 1971 Jul;107(1):114–120. doi: 10.1128/jb.107.1.114-120.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haidle C. W., Storck R. Control of dimorphism in Mucor rouxii. J Bacteriol. 1966 Oct;92(4):1236–1244. doi: 10.1128/jb.92.4.1236-1244.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. J. Aerobic microbial growth at low oxygen concentrations. J Bacteriol. 1967 Jul;94(1):101–108. doi: 10.1128/jb.94.1.101-108.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Rogers P. J., Stewart P. R. Mitochondrial and peroxisomal contributions to the energy metabolism of Saccharomyces cerevisiae in continuous culture. J Gen Microbiol. 1973 Dec;79(2):205–217. doi: 10.1099/00221287-79-2-205. [DOI] [PubMed] [Google Scholar]
- Rogers P. J., Stewart P. R. Respiratory development in Saccharomyces cerevisiae grown at controlled oxygen tension. J Bacteriol. 1973 Jul;115(1):88–97. doi: 10.1128/jb.115.1.88-97.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terenzi H. F., Storck R. Stimulation of fermentation and yeast-like morphogenesis in Mucor rouxii by phenethyl alcohol. J Bacteriol. 1969 Mar;97(3):1248–1261. doi: 10.1128/jb.97.3.1248-1261.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimpenny J. W., Necklen D. K. The redox environment and microbial physiology. I. The transition from anaerobiosis to aerobiosis in continuous cultures of facultative anaerobes. Biochim Biophys Acta. 1971 Dec 7;253(2):352–359. doi: 10.1016/0005-2728(71)90039-9. [DOI] [PubMed] [Google Scholar]
- Wright B. E. Multiple causes and controls in differentiation. Science. 1966 Aug 19;153(3738):830–837. doi: 10.1126/science.153.3738.830. [DOI] [PubMed] [Google Scholar]
- Zorzopulos J., Jobbagy A. J., Terenzi H. F. Effects of ethylenediaminetetraacetate and chloramphenicol on mitochondrial activity and morphogenesis in Mucor rouxii. J Bacteriol. 1973 Sep;115(3):1198–1204. doi: 10.1128/jb.115.3.1198-1204.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]