Abstract
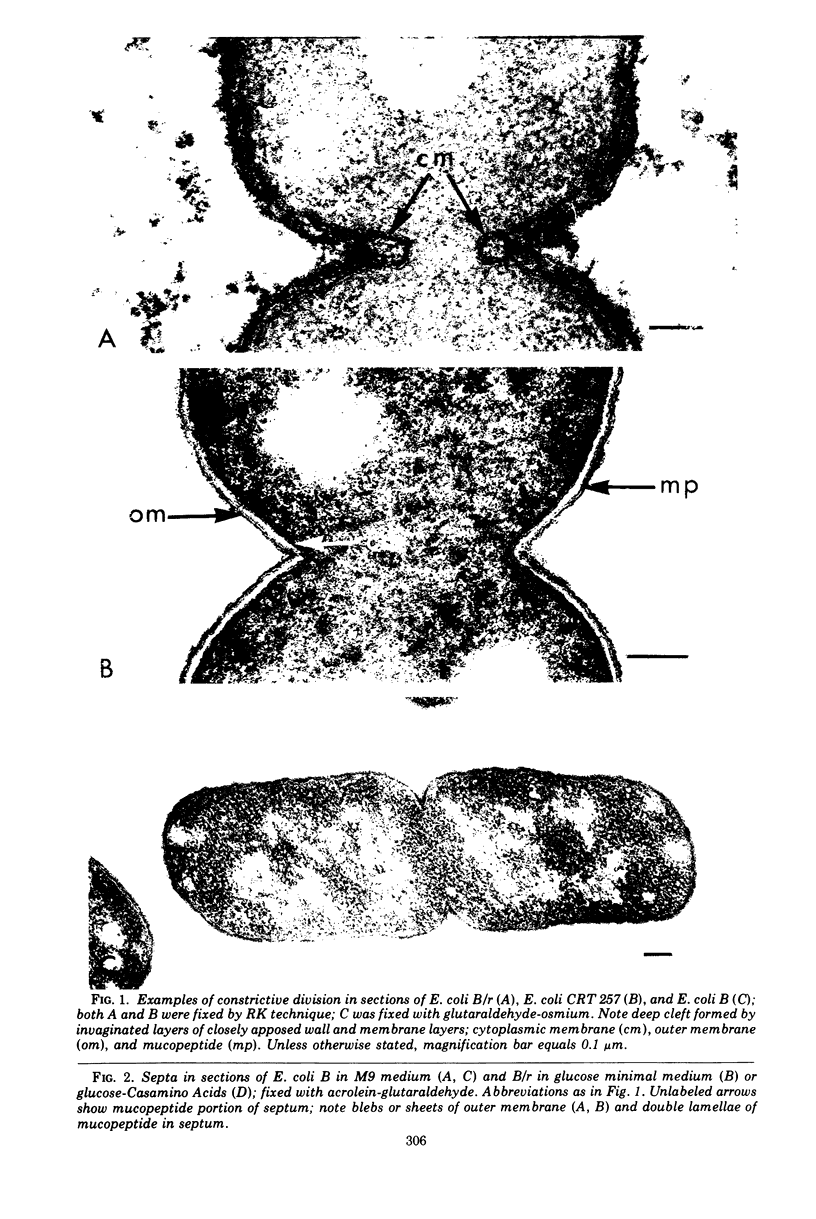
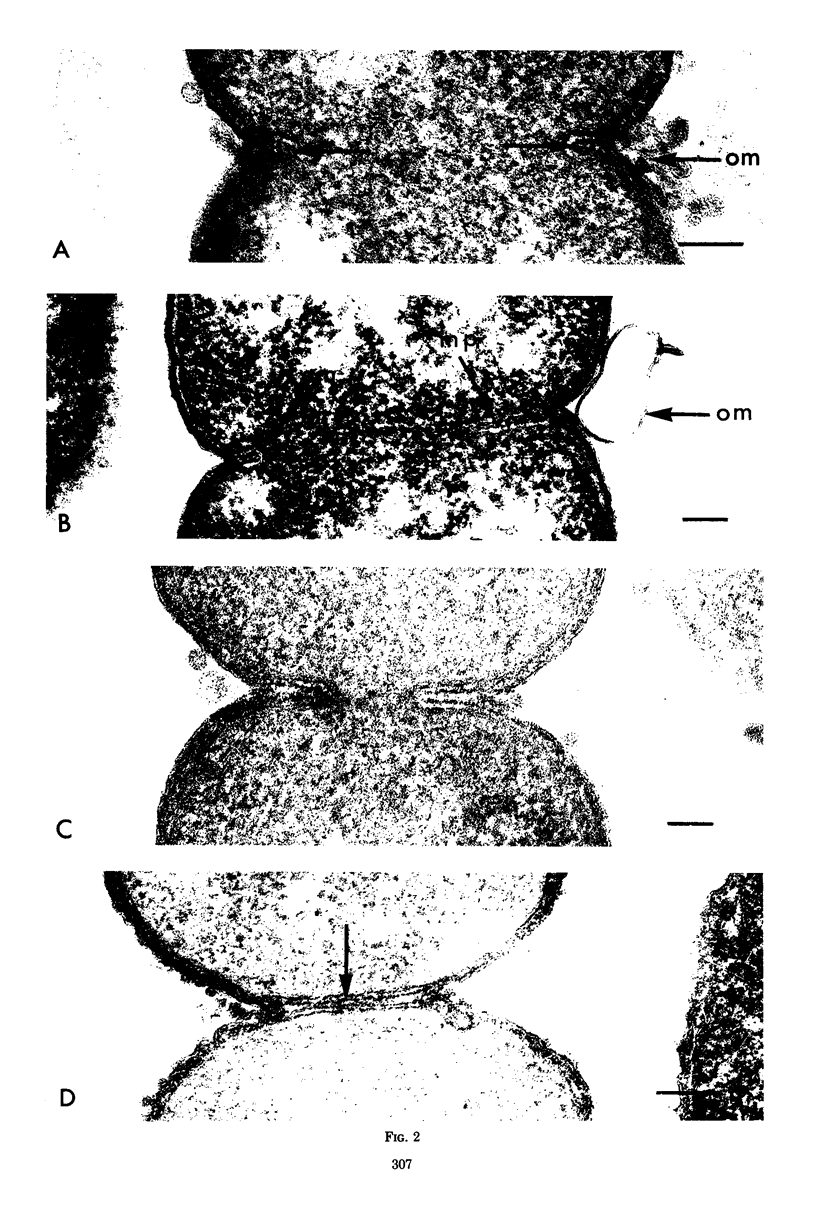
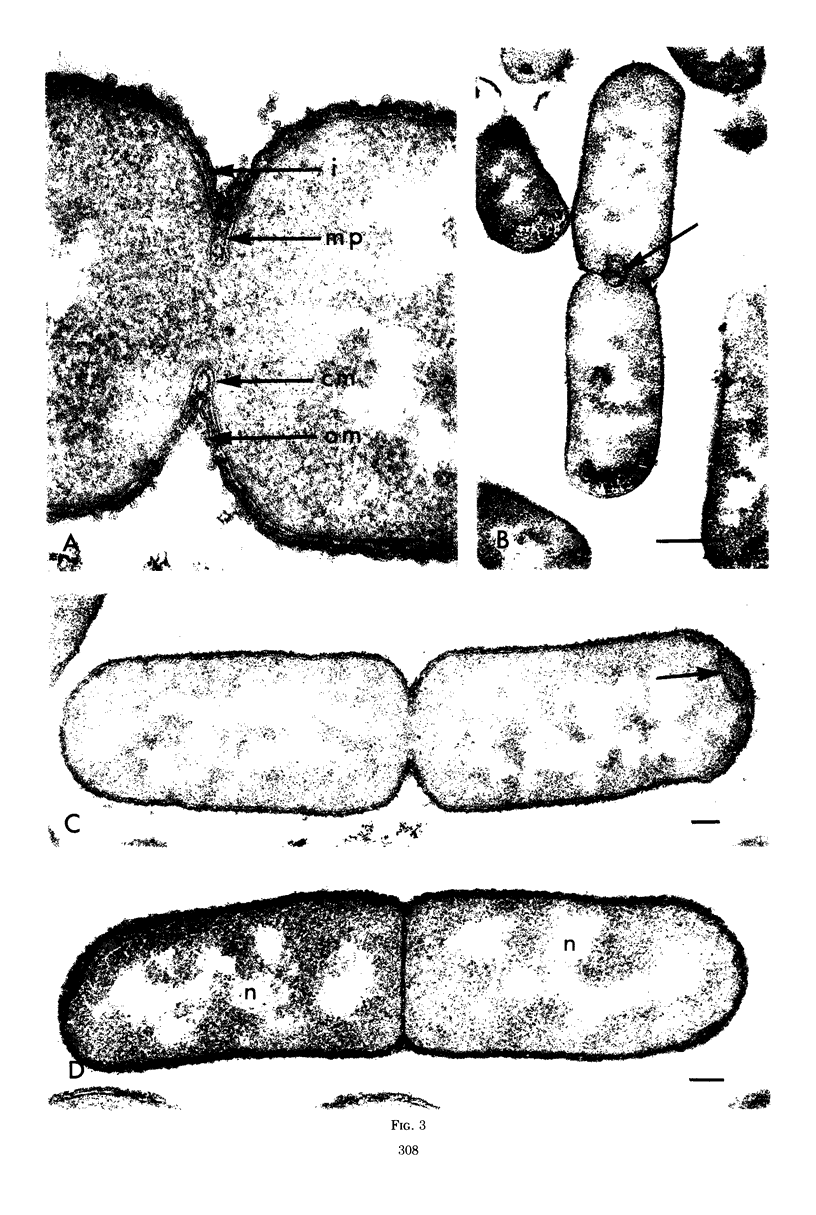
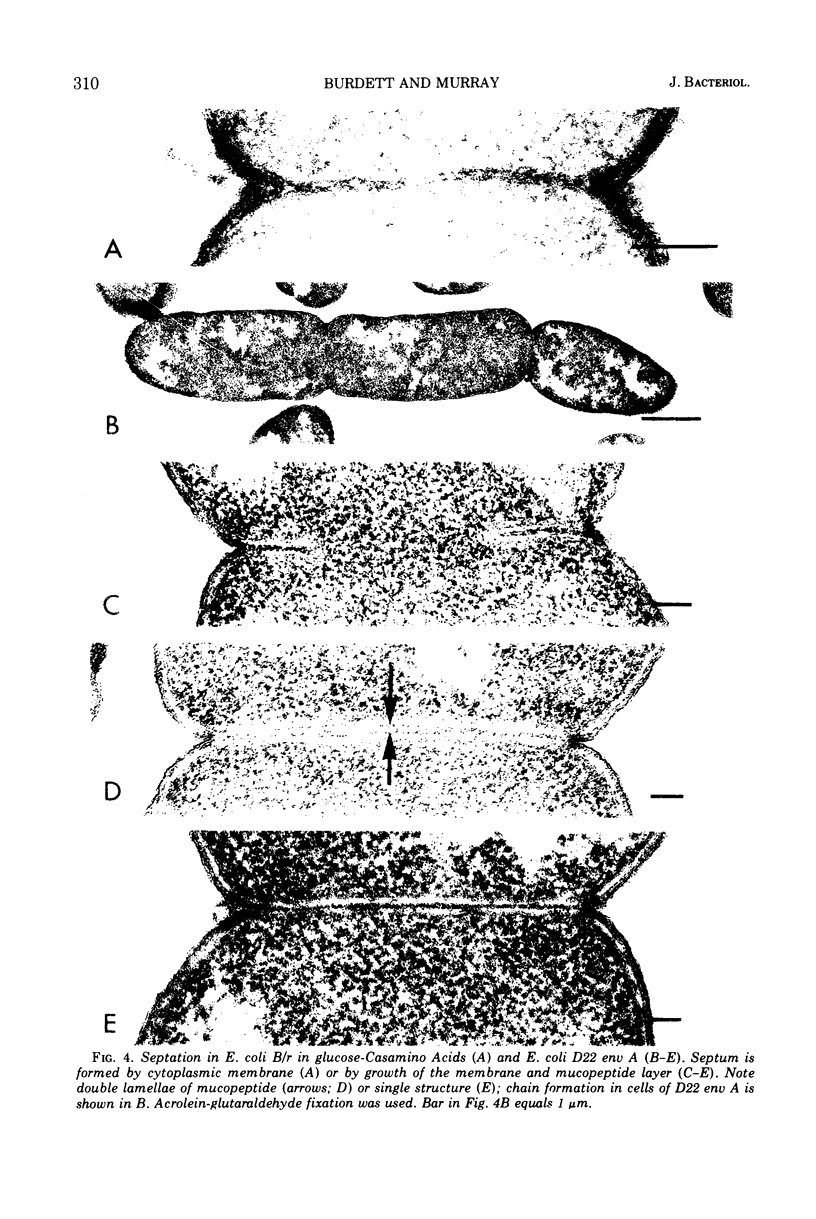
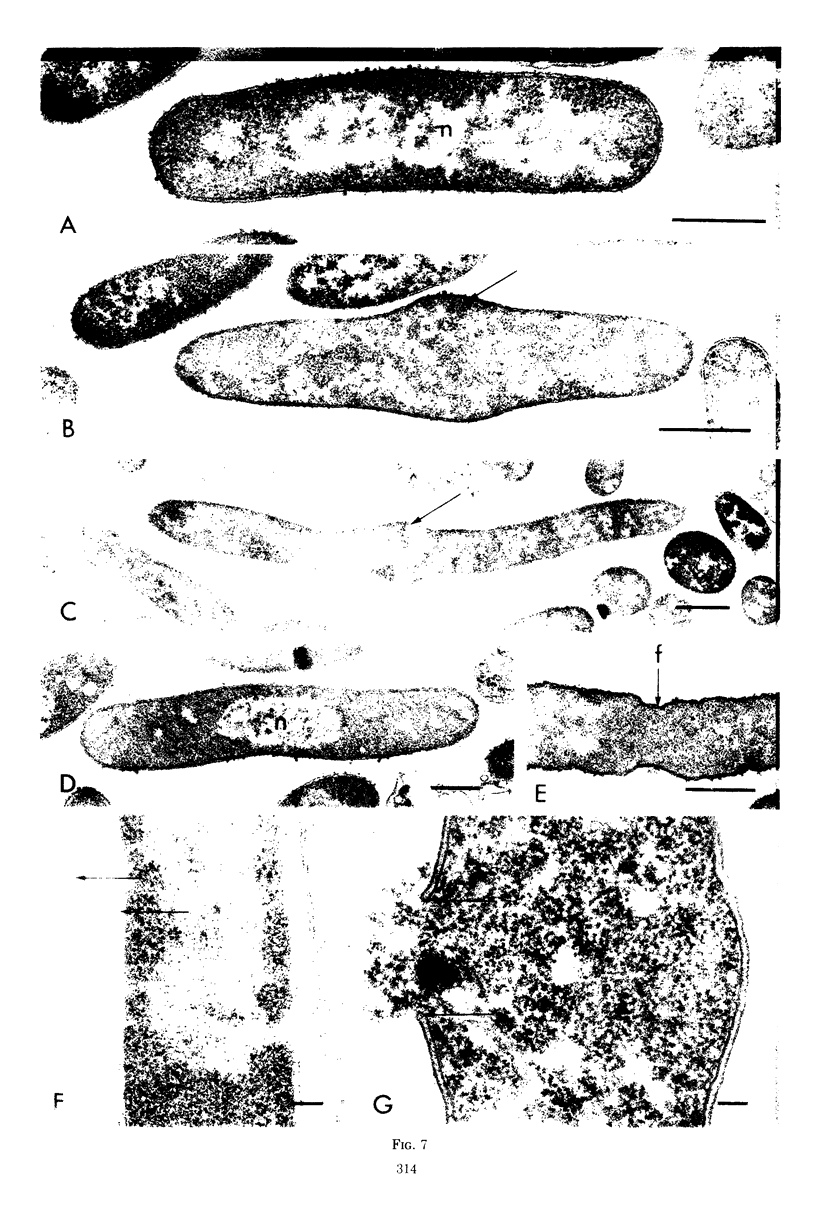
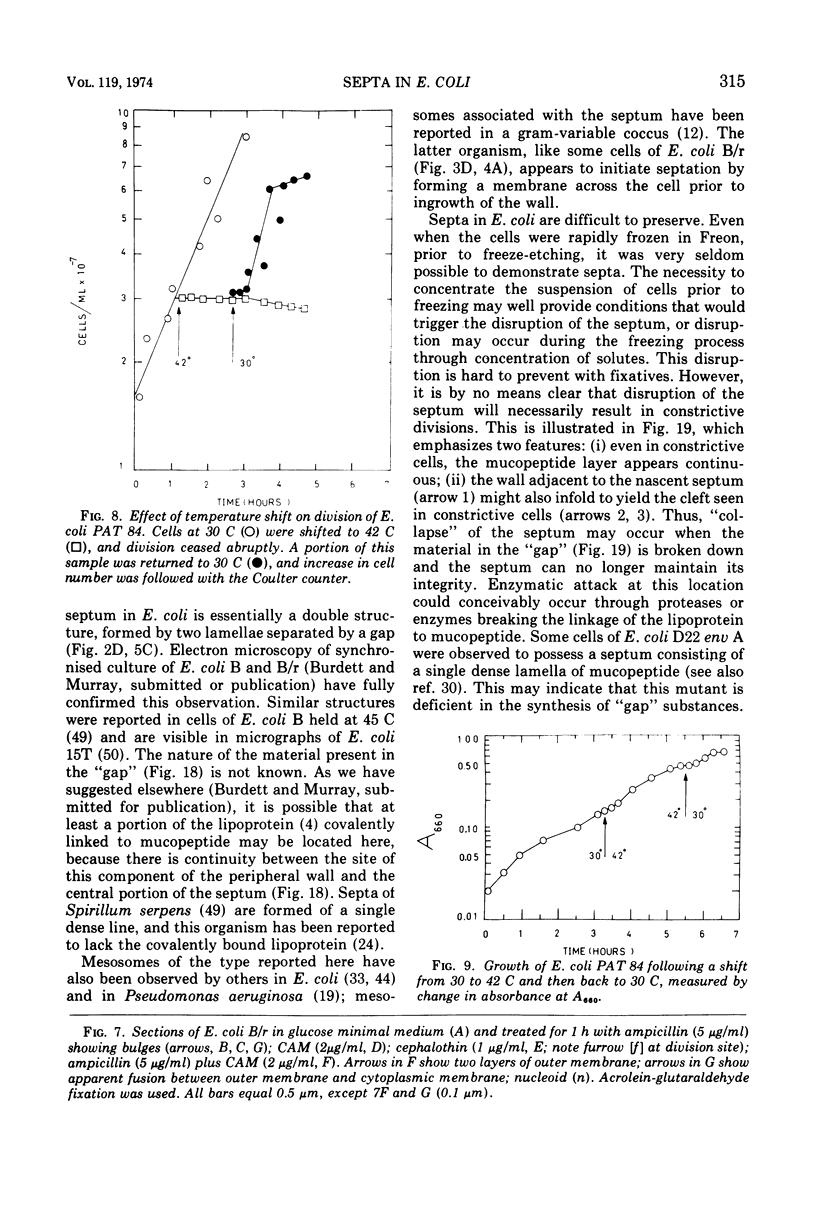
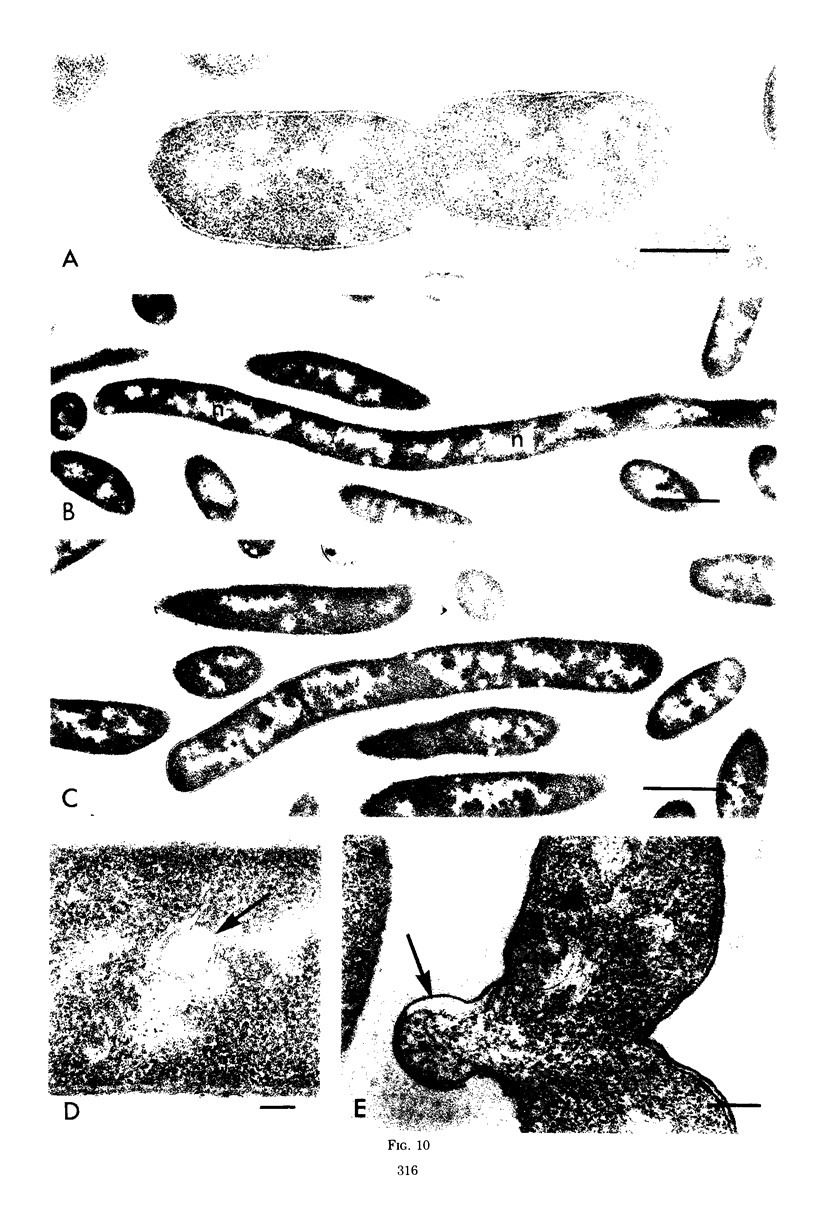
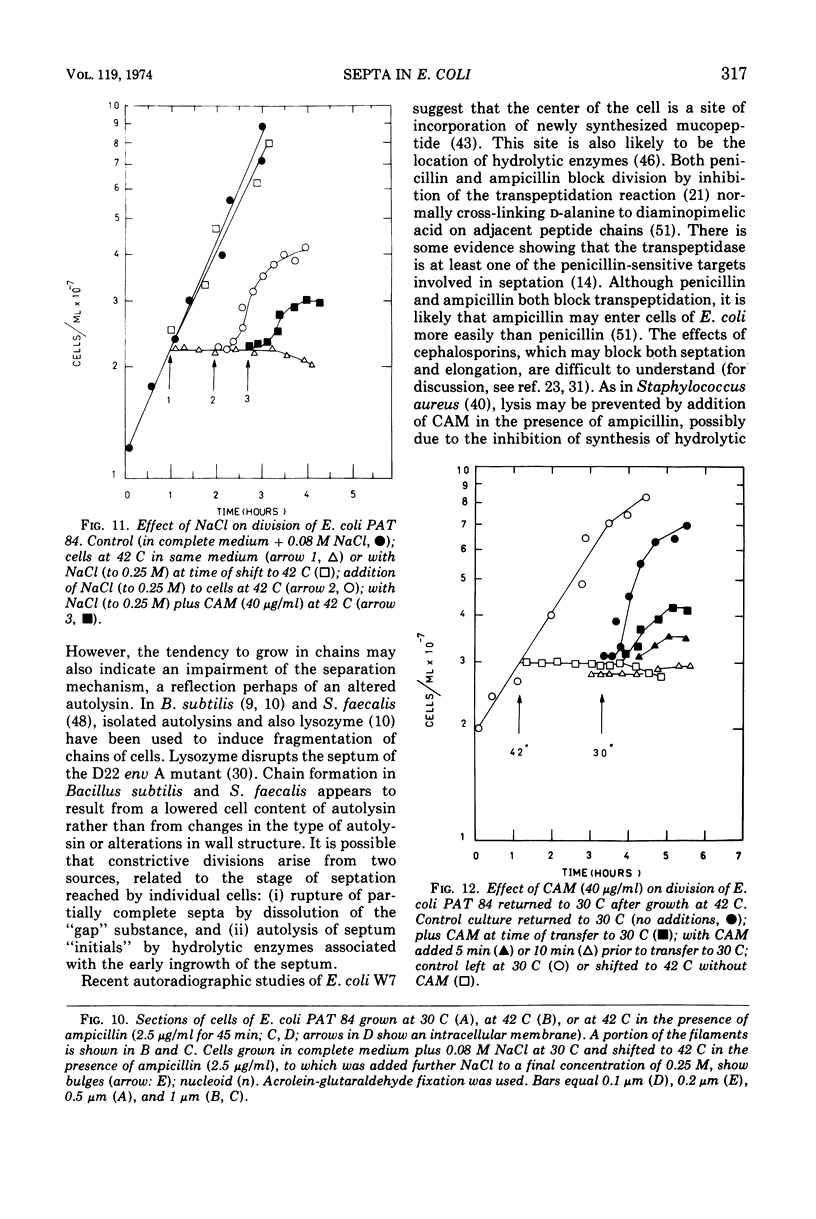
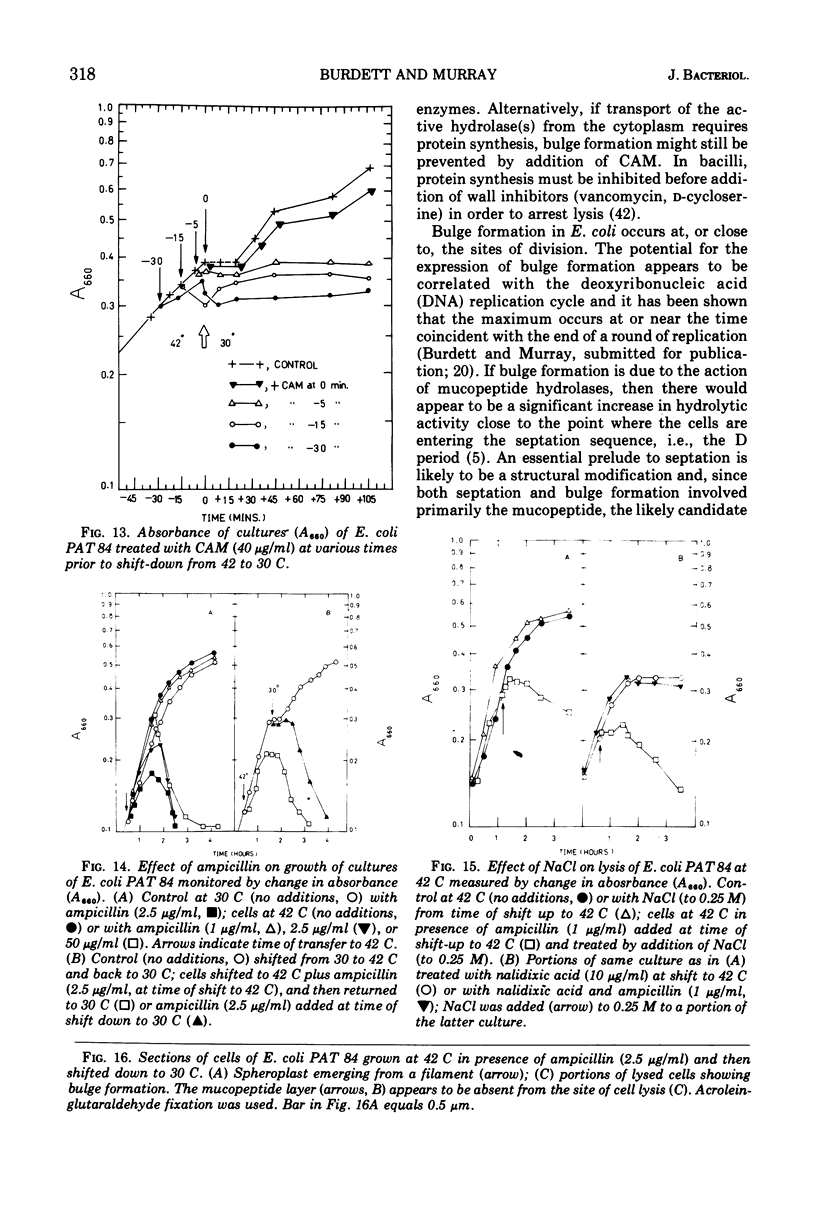
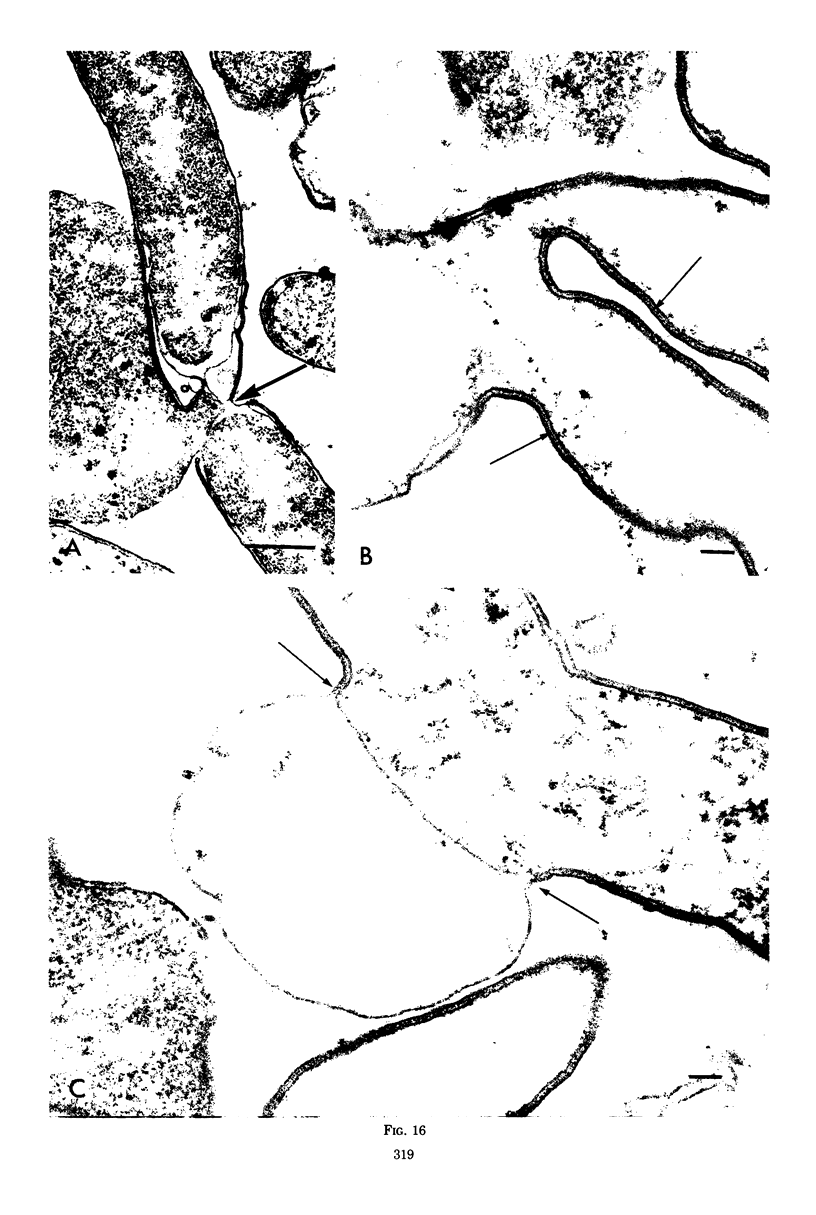
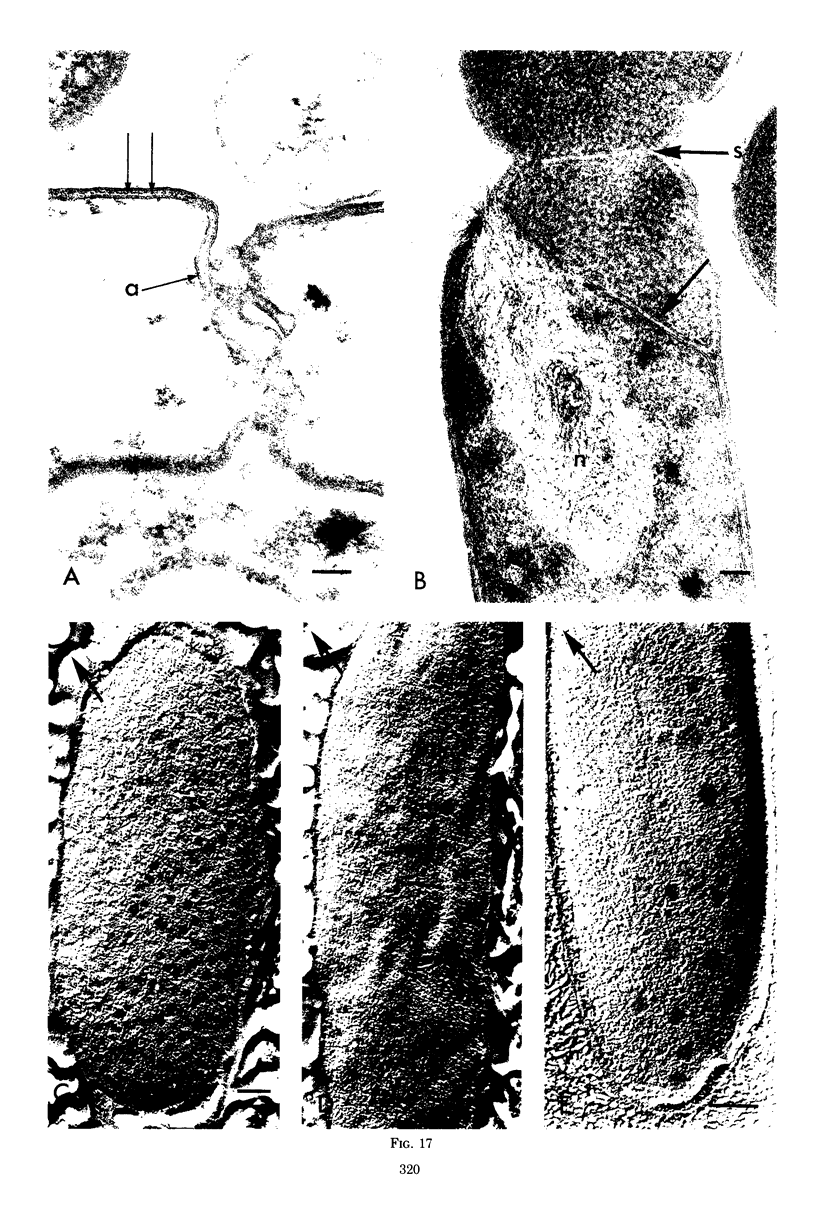
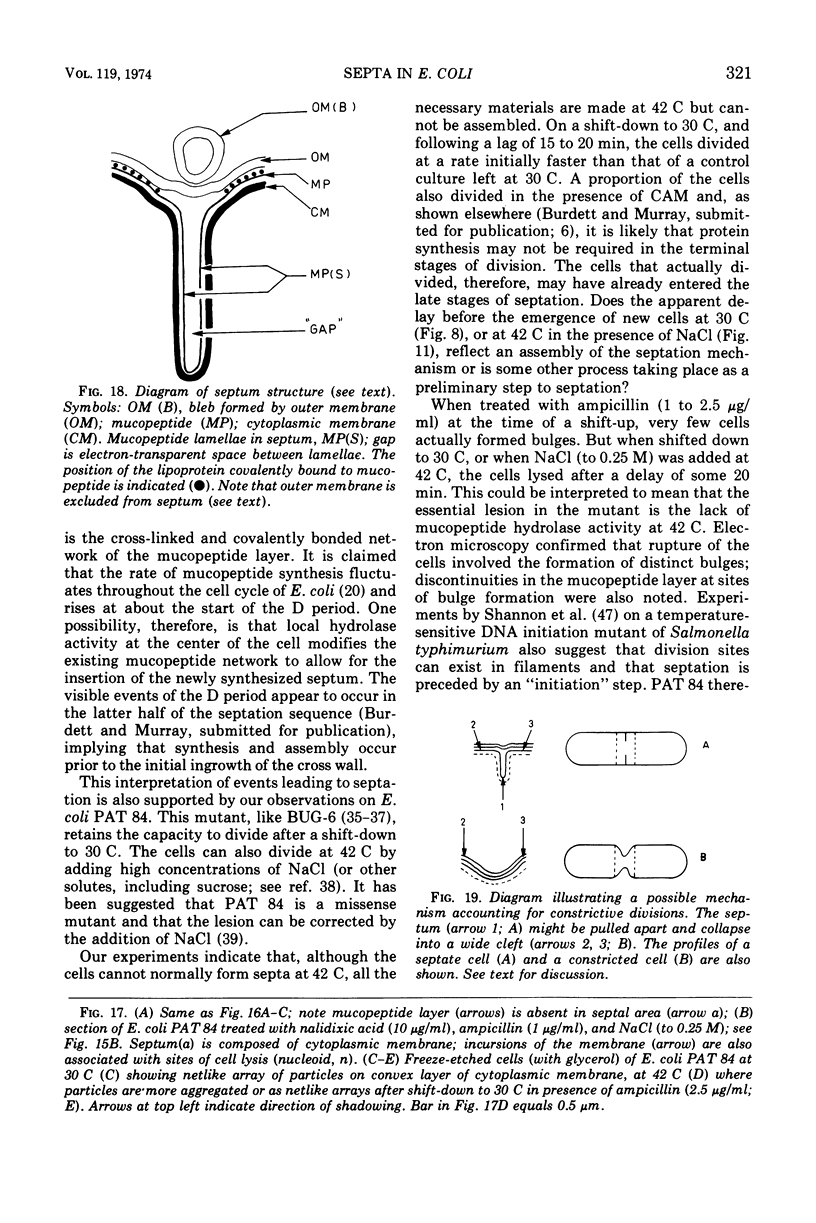
Septa can be demonstrated in sections of Escherichia coli strains B and B/r after fixation with acrolein and glutaraldehyde. The septum consists of an ingrowth of the cytoplasmic membrane and the mucopeptide layer; the outer membrane is excluded from the septum until the cells begin to separate. Mesosomes have also been observed. The septum is highly labile and, except in the chain-forming strains, E. coli D22 env A and CRT 97, not easily preserved by standard procedures. The labile nature of the septum may be due to the presence of autolysin(s) located at the presumptive division site. Blocking division by addition of ampicillin (2 to 5 μg/ml) to cells of E. coli B/r produces a bulge at the middle of the cells; bulge formation is stopped by addition of chloramphenicol. Cephalosporins also induce bulge formation but may stop cell elongation as well as division. Bulge formation, due to the presumed action of an autolysin(s), may be an initial step in the septation sequence when the mucopeptide is modified to allow construction of the septum. In a nonseptate filament-forming strain, PAT 84, which ceases to divide at 42 C, bulge formation only occurs in the presence of ampicillin at the time of a shift-down at 30 C or at 42 C in the presence of NaCl (0.25 to 0.34 M). Experiments with chloramphenicol suggest that the filaments are fully compartmentalized but fail to divide owing to the inactivation, rather than loss of synthesis, of an autolysin at 42 C.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ahmed N., Rowbury R. J. Antibiotics and cell division in a filament-forming mutant of Salmonella typhimurium. Microbios. 1971 Dec;4(15):181–191. [PubMed] [Google Scholar]
- BERTANI G. Studies on lysogenesis. I. The mode of phage liberation by lysogenic Escherichia coli. J Bacteriol. 1951 Sep;62(3):293–300. doi: 10.1128/jb.62.3.293-300.1951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Rehn K. Chemical characterization, spatial distribution and function of a lipoprotein (murein-lipoprotein) of the E. coli cell wall. The specific effect of trypsin on the membrane structure. Eur J Biochem. 1969 Oct;10(3):426–438. doi: 10.1111/j.1432-1033.1969.tb00707.x. [DOI] [PubMed] [Google Scholar]
- Cooper S., Helmstetter C. E. Chromosome replication and the division cycle of Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):519–540. doi: 10.1016/0022-2836(68)90425-7. [DOI] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- Dix D. E., Helmstetter C. E. Coupling between chromosome completion and cell division in Escherichia coli. J Bacteriol. 1973 Sep;115(3):786–795. doi: 10.1128/jb.115.3.786-795.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G., Slepecky R. A. Fine structure of Bacillus megaterium during synchronous growth. J Bacteriol. 1967 Oct;94(4):1189–1205. doi: 10.1128/jb.94.4.1189-1205.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P. Autolysin(s) of Bacillus subtilis as dechaining enzyme. J Bacteriol. 1970 Aug;103(2):494–499. doi: 10.1128/jb.103.2.494-499.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan D. P., Beckman M. M., Cunningham W. P. Ultrastructural studies on a mutant of Bacillus subtilis whose growth is inhibited due to insufficient autolysin production. J Bacteriol. 1972 Mar;109(3):1247–1257. doi: 10.1128/jb.109.3.1247-1257.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsberg C., Rogers H. J. Autolytic enzymes in growth of bacteria. Nature. 1971 Jan 22;229(5282):272–273. doi: 10.1038/229272a0. [DOI] [PubMed] [Google Scholar]
- Gilleland H. E., Jr, Roth I. L., Eagon R. G. Ultrastructure of the cell wall and the mechanism of cellular division of a gram-variable coccus. Can J Microbiol. 1971 Mar;17(3):421–424. doi: 10.1139/m71-069. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Hartmann R., Höltje J. V., Schwarz U. Targets of penicillin action in Escherichia coli. Nature. 1972 Feb 25;235(5339):426–429. doi: 10.1038/235426a0. [DOI] [PubMed] [Google Scholar]
- Helmstetter C. E. DNA synthesis during the division cycle of rapidly growing Escherichia coli B/r. J Mol Biol. 1968 Feb 14;31(3):507–518. doi: 10.1016/0022-2836(68)90424-5. [DOI] [PubMed] [Google Scholar]
- Higgins M. L., Pooley H. M., Shockman G. D. Site of initiation of cellular autolysis in Streptococcus faecalis as seen by electron microscopy. J Bacteriol. 1970 Aug;103(2):504–512. doi: 10.1128/jb.103.2.504-512.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins M. L., Shockman G. D. Procaryotic cell division with respect to wall and membranes. CRC Crit Rev Microbiol. 1971 May;1(1):29–72. doi: 10.3109/10408417109104477. [DOI] [PubMed] [Google Scholar]
- Hirota Y., Ryter A., Jacob F. Thermosensitive mutants of E. coli affected in the processes of DNA synthesis and cellular division. Cold Spring Harb Symp Quant Biol. 1968;33:677–693. doi: 10.1101/sqb.1968.033.01.077. [DOI] [PubMed] [Google Scholar]
- Hoffmann B., Messer W., Schwarz U. Regulation of polar cap formation in the life cycle of Escherichia coli. J Supramol Struct. 1972;1(1):29–37. doi: 10.1002/jss.400010105. [DOI] [PubMed] [Google Scholar]
- Hoffmann H. P., Geftic S. G., Heymann H., Adair F. W. Mesosomes in Pseudomonas aeruginosa. J Bacteriol. 1973 Apr;114(1):434–438. doi: 10.1128/jb.114.1.434-438.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Kohiyama M., Cousin D., Ryter A., Jacob F. Mutants thermosensibles d'Escherichia coli K 12. I. Isolement et caractérisation rapide. Ann Inst Pasteur (Paris) 1966 Apr;110(4):465–486. [PubMed] [Google Scholar]
- Lorain V., Sabath L. D. Penicillins and cephalosporins: differences in morphologic effects on Proteus mirabilis. J Infect Dis. 1972 May;125(5):560–564. doi: 10.1093/infdis/125.5.560. [DOI] [PubMed] [Google Scholar]
- MURRAY R. G., STEED P., ELSON H. E. THE LOCATION OF THE MUCOPEPTIDE IN SECTIONS OF THE CELL WALL OF ESCHERICHIA COLI AND OTHER GRAM-NEGATIVE BACTERIA. Can J Microbiol. 1965 Jun;11:547–560. doi: 10.1139/m65-072. [DOI] [PubMed] [Google Scholar]
- Martin H. H., Heilmann H. D., Preusser H. J. State of the rigid-layer in celll walls of some gram-negative Bacteria. Arch Mikrobiol. 1972;83(4):332–346. doi: 10.1007/BF00425246. [DOI] [PubMed] [Google Scholar]
- Moor H. Use of freeze-etching in the study of biological ultrastructure. Int Rev Exp Pathol. 1966;5:179–216. [PubMed] [Google Scholar]
- NAGATA T. The molecular synchrony and sequential replication of DNA in Escherichia coli. Proc Natl Acad Sci U S A. 1963 Apr;49:551–559. doi: 10.1073/pnas.49.4.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai K., Tamura G. Mutant of Escherichia coli with thermosensitive protein in the process of cellular division. J Bacteriol. 1972 Nov;112(2):959–966. doi: 10.1128/jb.112.2.959-966.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Normark S., Boman H. G., Bloom G. D. Cell division in a chain-forming envA mutant of Escherichia coli K12. Fine structure of division sites and effects of EDTA, lysozyme and ampicillin. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(5):651–664. doi: 10.1111/j.1699-0463.1971.tb00093.x. [DOI] [PubMed] [Google Scholar]
- Perkins R. L., Miller M. A. Scanning electron microscopy of morphological alterations in Proteus mirabilis induced by cephalosporins and semisynthetic penicillins. Can J Microbiol. 1973 Feb;19(2):251–255. doi: 10.1139/m73-038. [DOI] [PubMed] [Google Scholar]
- Pontefract R. D., Bergeron G., Thatcher F. S. Mesosomes in Escherichia coli. J Bacteriol. 1969 Jan;97(1):367–375. doi: 10.1128/jb.97.1.367-375.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E., BIRCHANDERSEN A., MAALOE O. Etude au microscope électronique de plasmas contenant de l'acide désoxyribonucliéique. I. Les nucléoides des bactéries en croissance active. Z Naturforsch B. 1958 Sep;13B(9):597–605. [PubMed] [Google Scholar]
- Reeve J. N., Clark D. J. Cell division of Escherichia coli BUG-6: effect of varying the length of growth at the nonpermissive temperature. J Bacteriol. 1972 Apr;110(1):117–121. doi: 10.1128/jb.110.1.117-121.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Clark D. J. Cell division of Escherichia coli BUG-6: effect of varying the temperature used as the nonpermissive growth condition. J Bacteriol. 1972 Apr;110(1):122–125. doi: 10.1128/jb.110.1.122-125.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve J. N., Groves D. J., Clark D. J. Regulation of Cell Division in Escherichia coli: Characterization of Temperature-Sensitive Division Mutants. J Bacteriol. 1970 Dec;104(3):1052–1064. doi: 10.1128/jb.104.3.1052-1064.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Effet des sels et autres composés sur le phénotype de mutants thermosensibles de Escherichia coli. Ann Microbiol (Paris) 1973 Jan;124(1):29–43. [PubMed] [Google Scholar]
- Ricard M., Hirota Y. Process of cellular division in Escherichia coli: physiological study on thermosensitive mutants defective in cell division. J Bacteriol. 1973 Oct;116(1):314–322. doi: 10.1128/jb.116.1.314-322.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodolakis A., Thomas P., Starka J. Morphological mutants of Escherichia coli. Isolation and ultrastructure of a chain-forming envC mutant. J Gen Microbiol. 1973 Apr;75(2):409–416. doi: 10.1099/00221287-75-2-409. [DOI] [PubMed] [Google Scholar]
- Rogers H. J. Bacterial growth and the cell envelope. Bacteriol Rev. 1970 Jun;34(2):194–214. doi: 10.1128/br.34.2.194-214.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers H. J., Forsberg C. W. Role of autolysins in the killing of bacteria by some bactericidal antibiotics. J Bacteriol. 1971 Dec;108(3):1235–1243. doi: 10.1128/jb.108.3.1235-1243.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryter A., Hirota Y., Schwarz U. Process of cellular division in Escherichia coli growth pattern of E. coli murein. J Mol Biol. 1973 Jun 25;78(1):185–195. doi: 10.1016/0022-2836(73)90437-3. [DOI] [PubMed] [Google Scholar]
- Ryter A., Jacob F. Etude morphologique de la liaison du noyau à la membrane chez E. coli et chez les protoplastes de B. subtilis. Ann Inst Pasteur (Paris) 1966 Jun;110(6):801–812. [PubMed] [Google Scholar]
- Schwarz U., Asmus A., Frank H. Autolytic enzymes and cell division of Escherichia coli. J Mol Biol. 1969 May 14;41(3):419–429. doi: 10.1016/0022-2836(69)90285-x. [DOI] [PubMed] [Google Scholar]
- Shannon K. P., Spratt B. G., Rowbury R. J. Cell division and the production of cells lacking nuclear bodies in a mutant of Salmonella typhimurium. Mol Gen Genet. 1972;118(2):185–197. doi: 10.1007/BF00267087. [DOI] [PubMed] [Google Scholar]
- Soper J. W., Winter C. G. Role of cell wall antolysin in chain formation by a mutant strain of Streptococcus faecalis. Biochim Biophys Acta. 1973 Feb 28;297(2):333–342. doi: 10.1016/0304-4165(73)90080-9. [DOI] [PubMed] [Google Scholar]
- Steed P., Murray R. G. The cell wall and cell division of gram-negative bacteria. Can J Microbiol. 1966 Apr;12(2):263–270. doi: 10.1139/m66-036. [DOI] [PubMed] [Google Scholar]
- Stevenson I. L. The cytological effects of myxin on Escherichia coli. Can J Microbiol. 1969 Jul;15(7):707–711. doi: 10.1139/m69-125. [DOI] [PubMed] [Google Scholar]
- Wu P. C., Pardee A. B. Cell division of Escherichia coli: control by membrane organization. J Bacteriol. 1973 May;114(2):603–611. doi: 10.1128/jb.114.2.603-611.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zusman D. R., Inouye M., Pardee A. B. Cell division in Escherichia coli: evidence for regulation of septation by effector molecules. J Mol Biol. 1972 Aug 14;69(1):119–136. doi: 10.1016/0022-2836(72)90027-7. [DOI] [PubMed] [Google Scholar]