Abstract
Strains of Bacteroides fragilis associated with diarrheal disease (enterotoxigenic B. fragilis) produce a 20-kDa zinc-dependent metalloprotease toxin (B. fragilis enterotoxin; BFT) that reversibly stimulates chloride secretion and alters tight junctional function in polarized intestinal epithelial cells. BFT alters cellular morphology and physiology most potently and rapidly when placed on the basolateral membrane of epithelial cells, suggesting that the cellular substrate for BFT may be present on this membrane. Herein, we demonstrate that BFT specifically cleaves within 1 min the extracellular domain of the zonula adherens protein, E-cadherin. Cleavage of E-cadherin by BFT is ATP-independent and essential to the morphologic and physiologic activity of BFT. However, the morphologic changes occurring in response to BFT are dependent on target-cell ATP. E-cadherin is shown here to be a cellular substrate for a bacterial toxin and represents the identification of a mechanism of action, cell-surface proteolytic activity, for a bacterial toxin.
Bacteroides fragilis is the leading cause of anaerobic bacteremia and intraabdominal abscess formation in humans (1). The major virulence determinant identified to date for B. fragilis is the capsular polysaccharide (2). Since 1984, strains of B. fragilis associated with diarrheal disease in animals and young children (termed enterotoxigenic B. fragilis or ETBF) have been recognized to secrete an ≈20-kDa protein toxin termed B. fragilis enterotoxin (BFT, also known as fragilysin; refs. 3–12). Sequencing of the bft gene, combined with substrate analysis in vitro, indicated that BFT could be classified as a zinc-dependent metalloprotease and revealed two isoforms of bft, bft1 and bft2 (13–15). ETBF strains produce one, but not both, of these two BFTs (14). In the studies performed to date, purified BFT-1 and BFT-2 have proven to be biochemically distinct proteins with concordant biological activities but differing potencies (S.W. and C.L.S., unpublished data). Both BFTs act in a reversible manner to alter the morphology and physiology of polarized epithelial cells, in particular human intestinal epithelial cells (HT29/C1, T84, normal human colon; refs. 7, 16, 17; M. Riegler, C.L.S., and C. Pothoulakis, unpublished data). In these cell types, actin rearrangement occurs without a quantitative change in total cellular actin (18); also, cell volume increases (18), resistance of polarized monolayers decreases (7, 16), and chloride secretion and protein synthesis are stimulated (16, 18). The activity of BFT on epithelial cell monolayers such as T84 cells (human colon carcinoma cell line) and normal human colon is polar, with greater biological activity demonstrable after application of BFT to the basolateral rather than to the apical cellular membranes (7, 16). Basolateral application of BFT produces striking apical-membrane morphologic changes with loss of the interdigitated microvillous membrane and apical actin staining but with increased actin identified in the basolateral domain of the monolayers (16). Although a primary site of action at the basolateral membrane is counterintuitive from a pathogenesis viewpoint, other enteric pathogens, namely Listeria monocytogenes, Yersinia spp., and Shigella spp., also have been identified to act predominantly from the basolateral membrane of intestinal epithelial cells, albeit after initial entry through M cells (19–21).
Polarity of intestinal epithelial cells derives from cell-surface signals and requires both cell–cell and cell–extracellular matrix (ECM) adhesion to create specialized membrane domains (22–25). Adhesion of the basal membrane protein, integrin, to the ECM is thought to initiate cellular polarization. The apical-membrane domain is separated from the lateral-membrane domain by the zonula occludens (“tight junction”) in which cell–cell adhesion is accomplished by the 65-kDa protein occludin (26). Adjacent to the zonula occludens is the zonula adherens, whose major structural protein is E-cadherin, a 120-kDa glycoprotein responsible for calcium-dependent, homotypic cell–cell adhesion (23, 27, 28). Both E-cadherin–E-cadherin and integrin–ECM adherence are required for development of full epithelial-cell polarity. The polarity of BFT’s action on intestinal epithelial cells and additional studies that indicated that BFT does not enter epithelial cells (7, 29, 30) led us to hypothesize that the cellular substrate for BFT lies on the basolateral membrane of polarized intestinal epithelial cells. Thus, to identify the cellular substrate for BFT, we examined the effect of BFT on adhesion proteins with extracellular domains presumably accessible to BFT’s protease activity.
METHODS
BFT Purification and HT29/C1 Biologic Assay.
BFT was purified from ETBF strain VPI13784 (BFT-1) or 86–5443-2–2 (BFT-2) as described (12, 16). The majority of the experiments were performed by using BFT-2, with key experiments replicated using BFT-1 (which yielded concordant results). Purified BFT was maintained at −70°C in a 0.05 M Tris/0.18 M NaCl buffer (pH 7.5) until use. The cellular activity of BFT was assessed by using the HT29/C1 cloned intestinal epithelial cell line as described (3, 29). To deplete intracellular ATP, HT29/C1 cells were treated with an uncoupler of oxidative phosphorylation, carbonyl cyanide m-chlorophenylhydrazone (2 μM; CCCP; Sigma) in glucose-free medium for 1 hr. Intracellular ATP was measured by using firefly luciferase and its substrate, luciferin (ATP determination kit, Molecular Probes).
Immunoblotting Experiments.
Immunoblotting (Western blotting) was performed as described by Sambrook et al. (31). Proteins separated by using SDS/PAGE were electrophoretically transferred to nitrocellulose membrane sheets (Bio-Rad). After blocking with 10% nonfat dry milk in Tris-buffered saline (TBS), the membrane was probed with the desired antibodies: (i) polyclonal occludin antibody (Zymed); (ii) polyclonal antibodies specific for the 25 terminal amino acids of the cytoplasmic domain of E-cadherin (E2 antibody) or α-catenin (gifts of James Nelson, Stanford University, Palo Alto, CA); (iii) monoclonal β-catenin antibody (Zymed); (iv) polyclonal zonula occluden-1 (ZO1) antibody (Zymed); or (v) mAbs to glyceraldehyde-3-phosphate dehydrogenase (GAPDH; a gift of Michael Sirover, Temple University, Philadelphia). Membranes were washed with TBS/0.1% Tween 20 and incubated with appropriate dilutions of secondary antibodies. After washing to remove unbound antibody, the membranes were developed by using the enhanced chemiluminescence system (Western blot chemiluminescence reagent, DuPont/NEN or Suprasignal chemiluminescent substrate, Pierce).
Immunofluorescent Confocal Microscopy.
HT29/C1 cells were treated with BFT (5 nM) for 30 or 60 min followed by fixation with 10% formaldehyde, immunostaining with either a rat IgG mAb to the extracellular domain of E-cadherin (Decma1 antibody, Sigma) or a polyclonal mouse anti-human β1-integrin IgG antibody (Upstate Biotechnology, Lake Placid, NY). E-cadherin and β1-integrin immunostaining were visualized by single- and dual-channel confocal immunofluorescent microscopy using Cy-2 anti-rat IgG- and Cy-5 anti-mouse IgG-conjugated second antibody fluorophores (Jackson ImmunoResearch). HT29/C1 cells were evaluated by using Nomarski optics in parallel with immunofluorescence.
Expression of E-cadherin in L cells.
L cells stably transfected with the E-cadherin gene under the control of a dexamethasone-inducible promoter were a gift of Tzuu-Shuh Jou and James Nelson (Stanford University, Palo Alto, CA). E-cadherin expression was induced by overnight treatment with 1 μM dexamethasone prior to use in biologic assays.
In Vitro Analyses.
The effect of BFT on E-cadherin in vitro was examined by using several protocols. First, HT29/C1 cells were removed from plastic by scraping into PBS with 2 mM phenylmethylsulfonyl fluoride (PMSF), which does not inhibit BFT (13), and sonicated, and a membrane fraction was prepared by centrifugation (2,000 × g, 15 min). Equal aliquots of the membrane fraction resuspended in HT29/C1 medium without serum were treated overnight with or without 25 nM BFT at 37°C. After centrifugation, the membrane was solubilized in SDS loading buffer and analyzed by Western blot using the E2 antibody as described above. Second, the extracellular domain of E-cadherin was affinity-purified from the culture supernatants of 293 cells stably transfected with an Epstein–Barr virus Ori-P-based vector containing a chimeric construct of the extracellular domain of E-cadherin fused with the human Ig Fc domain (Cad–Fc) under control of the human cytomegalovirus early promoter, as described by Chen and Nelson (32). Purified Cad–Fc was used experimentally as described in Results. Third, a 30-aa peptide spanning the C terminus of the extracellular domain plus the N-terminal transmembrane domain of of E-cadherin (NH2-VSVCDCEGAAGVCRKAQPVEAGLQIPAILG-COOH) was synthesized and purified by the Johns Hopkins University Peptide Synthesis Core by using established methods. This peptide (1 μg/μl) was treated with BFT (5–50 nM) in HT29/C1 cell medium without serum at 37°C for 1 hr followed by analysis by 16.5% Tris/Tricine⋅HCl gel electrophoresis (Bio-Rad).
Northern Blot and Reverse Transcription–Polymerase Chain Reaction (RT-PCR).
For Northern blot analysis, total RNA was extracted (Trizol Reagent; GIBCO/BRL) and poly(A)+ mRNA was isolated [Mini-Oligo(dT)-Cellulose spin column kit; 5 Prime → 3 Prime] from ≈107 control and BFT-treated cells (5 nM for 3, 24, or 48 hr). After electrophoresis, mRNA (5 μg) was transferred to nitrocellulose membranes. The membranes were prehybridized then hybridized at 42°C with a full-length α-32P-labeled-cDNA E-cadherin probe (labeled by using the Rapid Multiprime DNA labeling kit; Amersham). After washing under high-stringency conditions, membranes were examined by autoradiography. A PCR-generated probe for the glycolytic protein, glyceraldehyde-3-phosphate dehydrogenase (GAPDH), was used as a control to standarize the mRNA loading.
RT-PCR also was used to detect E-cadherin and actin expression in control HT29/C1 cells and in HT29/C1 cells treated with BFT (5 nM) for various times. Total RNA was isolated as described above and reverse-transcribed into cDNA by using the Superscript II Kit (Gibco/BRL). The PCR reaction to amplify the target cDNA was performed as described in the Superscript II Kit. To amplify E-cadherin and actin DNA, the following primers were used. For E-cadherin, a forward primer derived from the N terminus of the extracellular domain (5′-CAATCTCAAGCTCATGG) and a reverse primer derived from the C terminus of the cytoplasmic domain (5′-CCATTCGTTCAAGTAGTC); these primers generated a 600-bp fragment of E-cadherin DNA; for actin, forward primer 5′-ATGCCAACACAGTGCTGTCTGG and reverse primer 5′-TACTCCTGCTTGCTGATCCACAT) yielded a 210-bp product. For these PCR, initial denaturation was performed at 94°C for 4 min. Thirty amplification cycles were performed with denaturation at 94°C for 1 min, annealing at 55°C for 1 min, and extension at 72°C (40 sec in the first cycle with 1 additional second per subsequent cycle), followed by a final extension at 72°C for 10 min. PCR conditions were selected to permit detection of the PCR products in the linear range of the reaction. After electrophoresis, the gel was stained with ethidium bromide and photographed.
RESULTS
BFT Cleaves E-Cadherin.
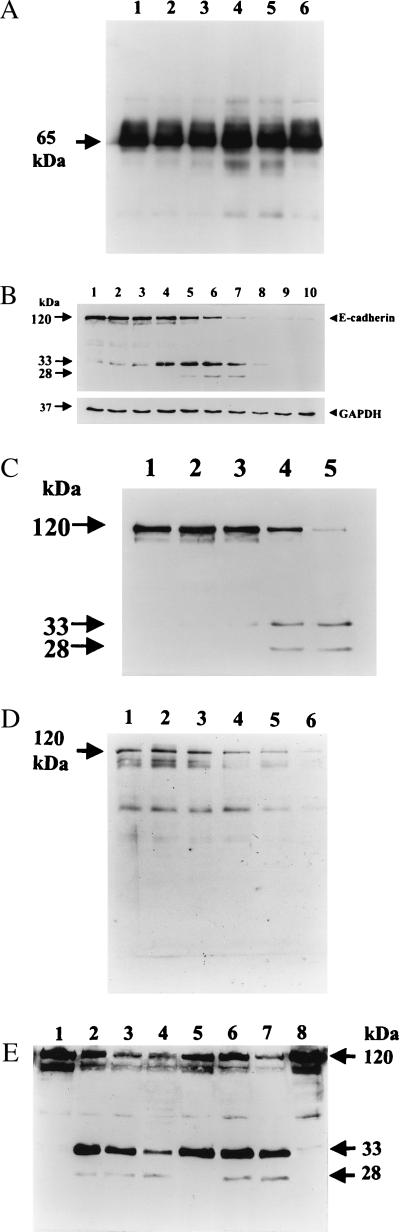
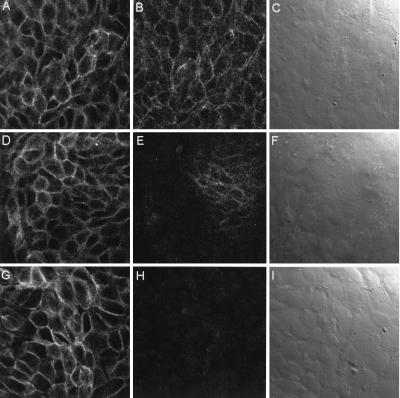
Our previous experiments (29) indicated that subconfluent HT29/C1 cells are exquisitely sensitive to BFT with 100 ng/ml (5 nM) BFT stimulating a change in cell morphology (including cell rounding and dissolution of tight clusters) in 100% of cells by 30–45 min. Thus, this cellular model of BFT activity was used to examine the effect of BFT on the epithelial-cell adhesion proteins occludin, β1-integrin, and E-cadherin. Using Western blot (Fig. 1A) analysis, occludin was not cleaved by treatment with BFT from 30 min to 3 hr, time points correlating with maximal changes in morphology stimulated by BFT. By using antibodies to β1-integrin and to the extracellular domain of E-cadherin (Decma antibody), HT29/C1 cells were examined by immunofluorescent confocal microscopy. Control cells revealed uniform membrane staining with both the anti- β1-integrin and E-cadherin antibodies (Fig. 2 A and B) and indistinct cell borders by Nomarski optics (Fig. 2C). After 30 min of BFT treatment (5 nM), cell-membrane E-cadherin staining was largely ablated, with complete loss of E-cadherin staining after 1 hr of BFT treatment (Fig. 2 E and H). In contrast, β1-integrin immunostaining was unaltered, as assessed by single- and dual-channel analysis over this time period (Fig. 2 D and G; data not shown). Parallel Nomarski analysis revealed increasingly distinct cell borders after BFT treatment of HT29/C1 cells (Fig. 2 F and I). These Nomarski observations are consistent with the diminished cell–cell contacts and increased cell volume previously reported in polarized and unpolarized intestinal epithelial cells after BFT treatment (7, 16–18, 33). Loss of E-cadherin staining after BFT treatment could indicate either that E-cadherin was redistributed in the cell or that the protein was destroyed proteolytically by BFT. In contrast, these data indicate that neither β1-integrin nor occludin was cleaved by BFT.
Figure 1.
Cleavage of E-cadherin, but not occludin, by BFT. (A) Occludin immunoblot of HT29/C1 cells. HT29/C1 cells were treated with BFT (5 nM) for various times, lysed, and examined by Western blotting using a polyclonal anti-human occludin antibody. Occludin is a 65-kDa protein (arrow). Lane 1, untreated HT29/C1 cells; lanes 2–5, BFT for 30 min, 60 min, 2 hr, 3 hr, respectively; lane 6, untreated HT29/C1 cells. (B) Time course of BFT proteolysis of E-cadherin. HT29/C1 cells were treated for various times with BFT (5 nM), lysed in 1× SDS/gel loading buffer, and examined by Western blot using the E2 antibody (34). Cell-shape changes were observed beginning 10 min after treatment with BFT, with 100% of cells affected by 30 min. Arrows indicate intact E-cadherin (120 kDa) and E-cadherin fragments (33 and 28 kDa) observed after BFT treatment. Lane 1, untreated HT29/C1 cells; lanes 2–10, BFT for 1 min, 3 min, 5 min, 10 min, 15 min, 30 min, 1 hr, 2 h, 3 hr, respectively. Immunostaining of the housekeeping protein GAPDH showed no change over time. (C) Concentration dependency of proteolysis of E-cadherin by BFT. HT29/C1 cells were treated with 0.005–5 nM BFT for 30 min. The immunoblot was processed and probed with E2 antibody as in 1B. Lane 1, untreated HT29/C1 cells; lane 2, 0.005 nM BFT; lane 3, 0.05 nM BFT; lane 4, 0.5 nM BFT; lane 5, 5 nM BFT. (D) Time course of BFT proteolysis of E-cadherin expressed by LE cells. LE cells (E-cadherin-transfected L cells) were induced overnight with 1 μM dexamethasone followed by treatment for various times with BFT (5 nM). The immunoblot was processed and probed with the E2 antibody as in Fig. 1B. Lane 1, control LE cells; lanes 2–6, BFT for 5 min, 10 min, 15 min, 30 min, 1 hr, respectively. (E) Effect of CCCP on BFT-stimulated E-cadherin proteolysis. HT29/C1 cells were treated with CCCP as described in Materials and Methods. CCCP-treated HT29/C1 cells were compared with HT29/C1 cells treated with BFT (5 nM) in standard HT29/C1 medium. Lanes 1–4, HT29/C1 cells without CCCP treatment. Lane 1, control HT29/C1 cells; lanes 2–4, BFT for 10 min, 30 min, 60 min, respectively. Lanes 5–8, HT29/C1 cells treated with CCCP. Lane 5, BFT for 10 min, 30 min, 60 min, control HT29/C1 cells, respectively.
Figure 2.
Immunofluorescent confocal microscopy of BFT-treated HT29/C1 cells. (A) Control β1-integrin. (B) Control E-cadherin. (C) Control Nomarski optics. Edges of individual control (untreated) cells are indistinct by Nomarski optics. (D) Thirty minutes after BFT-β1-integrin treatment. (E) Thirty minutes after BFT-E-cadherin treatment. (F) Thirty-minute BFT-Nomarski optics. Note the dramatic loss of E-cadherin immunofluorescence without a change in β1-integrin immunofluorescence. By Nomarski optics, HT29/C1 cellular borders are more distinct in areas of loss of E-cadherin immunofluorescence but remain indistinct where E-cadherin immunofluorescence remains intact. (G) Sixty minutes after BFT- β1-integrin treatment. (H) Sixty minutes after BFT-E-cadherin treatment. (I) Sixty-minute BFT-Nomarski optics. By Nomarski optics, individual HT29/C1 cell borders are visible. (Magnification, ×1,200.)
To address whether E-cadherin degradation or cellular redistribution was the mechanism for loss of the E-cadherin staining identified by confocal immunofluorescent microscopy, Western blot analysis using an antibody specific for the terminal 25 amino acids of the cytoplasmic domain of E-cadherin (E2 antibody) was used to analyze the E-cadherin content of BFT-treated cells (ref. 34; Fig. 1B). In these experiments, HT29/C1 morphologic changes were first observed 10 min after BFT treatment (5 nM), with 100% of the cells affected by 30 min. Using the E2 antibody, a 33-kDa fragment of E-cadherin was detected by Western blot at 1 min after BFT treatment (Fig. 1B). After 10 min of BFT treatment, a second 28-kDa E-cadherin cleavage fragment was detected (Fig. 1B). After 15–30 min of BFT treatment, disappearance of intact E-cadherin correlated with intensification of the 28- and 33-kDa bands. Subsequently, loss of immunostaining of both proteolytic cleavage products occurred. Additional experiments revealed that the 28- and 33-kDa fragments could also be immunoprecipitated with the E2 antibody (data not shown) but, to date, these fragments have not been isolated in sufficient quantity for N-terminal amino acid sequencing. However, the combined intracellular and transmembrane domains of E-cadherin are predicted to be ≈30 kDa (23, 27, 28), suggesting that the cleavage of E-cadherin by BFT observed in these experiments occurs near the plasma membrane. Concentration-dependency experiments performed at 30 min (Fig. 1C) and 3 hr (data not shown) after BFT treatment revealed detectable cleavage of E-cadherin with 0.5 nM BFT at 30 min (a concentration altering the morphology of 25% of cells) and 0.05 nM BFT at 3 hr (correlating with 75% change in cell morphology). We concluded from these experiments that BFT rapidly cleaves the extracellular domain of E-cadherin.
E-Cadherin Proteolysis Is a Two-Step ATP-Independent and -Dependent Event.
We next hypothesized that the degradation of the 28- to 33-kDa E-cadherin fragments observed 60 minutes or later after BFT treatment of HT29/C1 cells (Fig. 1B, Lanes 7–9) was caused by cellular proteases because available data indicate that BFT does not enter cells (7, 29, 30). To test this hypothesis, HT29/C1 cells were treated with an uncoupler of oxidative phosphorylation, carbonyl cyanide m-chlorophenylhydrazone (CCCP, 2 μM), in glucose-free medium for 1 hr to deplete cellular ATP and inhibit cellular enzymes (35). We reasoned that if BFT directly cleaved the extracellular domain of E-cadherin, cellular ATP depletion would not alter this activity. Alternatively, if cell entry of BFT or another cellular protease (potentially activated by BFT) was necessary for the cleavage of E-cadherin, cellular ATP depletion may inhibit the activity of BFT. Treatment of HT29/C1 cells with 2 μM CCCP decreased cellular ATP levels by ≈80% (data not shown). Under these conditions, E-cadherin was cleaved by BFT similarly to HT29/C1 cells treated with BFT in the absence of CCCP but, in the CCCP-treated cells, neither the 33- nor the 28-kDa E-cadherin fragments were further degraded over time (Fig. 1D, lane 7 vs. lane 4). Notably, HT29/C1 cells treated with BFT in normal medium (without CCCP) changed shape over the 1-hr time course, whereas no morphologic changes were observed in ATP-depleted HT29/C1 cells. After washing to remove the CCCP, cellular ATP levels recovered within 30–60 min, consistent with prior reports of the rapid reversibility of ATP depletion (data not shown; ref. 35). Concomitantly, after removal of the CCCP, the BFT-treated HT29/C1 cells developed typical morphologic changes, and subsequent degradation of the 28- and 33-kDa E-cadherin fragments occurred.
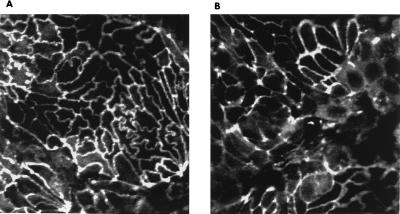
Consistent with our hypothesis that BFT acts only from the cell surface, no changes in the intracellular proteins, β-catenin, α-catenin, or ZO-1, were identified by Western blot over a 1-hr time course when BFT-treated HT29/C1 cells were compared with untreated control cells (data not shown). However, analysis of the cellular distribution of ZO1 in polarized HT29/C1 cells by confocal immunofluorescent microscopy revealed an even membrane distribution of the protein in control cells but a diffuse and punctate distribution after a 5 nM BFT treatment for 1 hr (Fig. 3). Parallel analysis revealed a similar change in the localization of occludin at 60 min after BFT treatment (data not shown).
Figure 3.
ZO1 redistribution in HT29/C1 cells after BFT treatment. Polarized HT29/C1 cells were treated with BFT (5 nM) for 1 hr and assessed by confocal immunofluorescent microscopy. (A) Control cells reveal the even membrane distribution of ZO1. (B) ZO1 immunofluorescence is more diffuse and punctate after BFT treatment. (Magnification, ×1,000.)
Effect of BFT on Expressed E-Cadherin.
To further examine whether BFT acts directly on E-cadherin, E-cadherin membrane expression was induced in L cells, a fibroblast cell line that does not express E-cadherin. This experiment revealed that BFT again cleaved E-cadherin (Fig. 1E) but with two fundamental differences when compared with HT29/C1 cells (Fig. 1B). First, although the immunoblot was analyzed by using the E2 antibody, discrete cleavage products were not observed (rather, only loss of intact E-cadherin by Western blot occurred, as shown); second, BFT treatment did not result in any change in cell shape when examined at time points up to 24 hr (data not shown).
Cellular Recovery After BFT Correlates with Resynthesis of E-Cadherin.
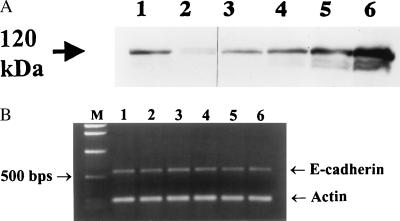
Prior observations indicate that the BFT effect on HT29/C1 cells does not induce cell death (7, 16, 18), that HT29/C1 cellular protein synthesis is stimulated beginning 5 hr after BFT treatment (16, 18), and that HT29/C1 cells recover normal morphology within 2–3 days of BFT treatment (29, 33). To examine the relationship between E-cadherin cleavage by BFT and cell recovery, HT29/C1 cells were treated with BFT for 3 hr, washed, and examined morphologically and by Western blot by using the E2 antibody at different time points up to 48 hr later (Fig. 4A). Morphologic observations revealed that 100% of cells had altered morphology by 3 hr after BFT treatment, with a gradual return of normal cellular morphology by 48 hr. Analysis of E-cadherin revealed nearly absent E-cadherin immunostaining after 3 hr of BFT treatment (Fig. 4A) with initial resynthesis detected at 12 hr (data not shown) and nearly complete resynthesis detected by 48 hr (Fig. 4A). To assess whether transcriptional activation of the E-cadherin gene accompanied the resynthesis of E-cadherin after BFT treatment, Northern blot analysis and RT-PCR were used to detect expression of the E-cadherin gene. No increase in E-cadherin transcript level was detected at 3 or 48 hr after BFT treatment when HT29/C1 cellular mRNA was probed by Northern blot (data not shown) or at time points of 1–24 hr after BFT treatment of HT29/C1 cells by RT-PCR (Fig. 4B).
Figure 4.
E-cadherin expression in recovering BFT-treated HT29/C1 cells. HT29/C1 cells were treated with BFT (5 nM) for 3 hr, washed, placed in normal medium, and assessed at various times. (A) Western blot of E-cadherin changes over time after BFT treatment. After 3 hr of BFT treatment, all cells exhibited altered morphology. Cellular morphology gradually returned to normal by 45 hr after washing to remove BFT from the cells. At each time point, cell lysates were examined by Western blot using the E2 antibody as described in Fig. 1B. Lane 1, 3-hr control HT29/C1 cells; lane 2, BFT for 3 hr; lane 3, 19 hr after removal of BFT; lane 4, 33 hr after removal of BFT; lane 5, 45 hr after removal of BFT; lane 6, 48-hr control HT29/C1 cells. (B) Analysis of E-cadherin expression. Lane 1, untreated HT29/C1 cells; lanes 2–6, BFT for 1 hr, 3 hr, 6 hr, 10 hr, and 24 hr, respectively.
Effect of BFT on E-Cadherin in Vitro.
Several approaches (see Materials and Methods) were utilized to assess whether cleavage of E-cadherin by BFT could be detected in vitro. These included examination by Western blot analysis of isolated membranes of HT29/C1 cells treated with BFT and treatment of the purified Cad–Fc fusion protein with BFT (5–50 nM for 1–24 hr) in HT29/C1 cell medium or after attachment of Cad–Fc to pansorbin cells or to agarose beads. No cleavage of native E-cadherin or Cad–Fc was observed under these conditions or by addition of soluble or insoluble cell fractions or whole-cell lysates to Cad–Fc. Because the expressed extracellular domain of E-cadherin was derived from Madin–Darby canine kidney (MDCK) cells, the activity of BFT on MDCK cells was examined. BFT treatment of the basolateral membranes of polarized MDCK cells resulted in E-cadherin cleavage as observed for HT29/C1 cells, indicating that the activity of BFT on E-cadherin was not species-specific (data not shown). Last, because the site of E-cadherin cleavage by BFT is predicted to be near the cell membrane (based on the size of the cleaved E-cadherin fragments detected by Western blot; Fig. 1B) and because the fusion peptide lacked the C-terminal 11 amino acids of the extracellular domain of E-cadherin treatment, a 30-aa peptide representing this region and its flanking amino acids was synthesized and treated with BFT. No cleavage of this peptide by BFT was detected. We also examined whether the mAb to the extracellular domain of E-cadherin, the Cad–Fc fusion protein, or the synthetic E-cadherin peptide could inhibit the action of BFT on HT29/C1 cells. No obvious inhibition was observed under any of the conditions tested.
DISCUSSION
These experiments indicate that BFT rapidly cleaves the extracellular domain of the zonula adherens protein, E-cadherin, in an ATP-independent manner. In contrast, the cellular morphologic changes precipitated by BFT are ATP-dependent. Consistent with our data that cleavage of E-cadherin (in the presence of ATP) is essential to the morphologic changes stimulated by BFT, the recovery of HT29/C1 cells after BFT treatment correlates with the resynthesis of E-cadherin. Alternatively, BFT could activate another cell-surface protease, which subsequently cleaves E-cadherin. However, this is unlikely given the proteolytic effect of BFT on E-cadherin in ATP-depleted HT29/C1 cells and the cleavage by BFT of E-cadherin expressed in L cells, which are unlikely to express accessory cell-surface proteins identical to polarizable epithelial cells. These latter data further indicate that the biological activity of BFT is epithelial cell-specific, consistent with previous reports (4, 33). Our inability to identify cleavage of E-cadherin by using several in vitro approaches suggests either that the native conformation of E-cadherin on epithelial cells is critical to observe BFT’s activity or that the Fc domain of the Cad–Fc fusion protein sterically hinders the activity of BFT.
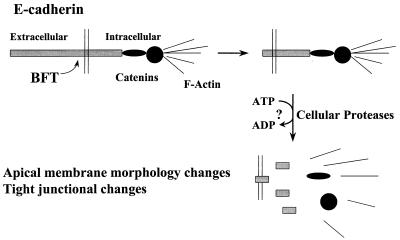
Stabilization of the 28- and 33-kDa E-cadherin cleavage products in ATP-depleted cells followed by the subsequent loss of these fragments after ATP repletion are consistent with the hypothesis that the BFT-generated E-cadherin remnants are degraded by cellular proteases. Proteolysis of the intracellular domain of E-cadherin would be predicted to disrupt the association of E-cadherin with β-catenin, which links E-cadherin to α-catenin and hence actin (36, 37), precipitating the characteristic apical-membrane morphology changes of polarized epithelial cells caused by BFT (16) and the reported dysregulation (reduced epithelial-cell monolayer resistance) and dissolution of the zonula occludens (refs. 7, 16; M. Riegler, C.L.S., and C. Pothoulakis, unpublished data). Alternatively, disruption of the zonula adherens by BFT may trigger a cell-signaling cascade that alters the function of the zonula occludens (44). Together these data suggest a model for the initial steps in the mechanism of action of BFT (Fig. 5).
Figure 5.
Model of BFT action. BFT cleaves the extracellular domain of the zonula adherens protein, E-cadherin, in an ATP-independent manner. Removal of the extracellular domain of E-cadherin results in the ATP-dependent proteolysis of the intracellular domain of E-cadherin, most likely by cellular proteases. Loss of intact E-cadherin disrupts its linkages with β-catenin and secondarily α-catenin and actin, leading to the characteristic disruption of the apical cytoskeleton of polarized epithelial cells as previously reported (16).
This report identifies E-cadherin as a cellular substrate for a bacterial toxin and represents the identification of a heretofore unrecognized mechanism by which a bacterial toxin stimulates intestinal secretion (16, 38) and alters the cytoskeletal structure of epithelial cells by cleavage of a cell-surface protein. In contrast, the only well-defined substrate-specific protease toxins described to date—tetanus and botulinum toxins—act intracellularly (39, 40). Similarly, although numerous cytoskeleton-altering enteric bacterial toxins have been described (41), the mechanism of action of only select clostridial toxins associated with intestinal disease have been determined; these toxins, in contrast to BFT, act intracellularly by either monoglucosylation of the small GTP-binding protein, Rho (Clostridium difficile toxins A and B) or by ADP-ribosylation of actin (C. botulinum C2 toxin; ref. 41). Of interest, E-cadherin also has been described as one potential ligand utilized by L. monocytogenes to enter cells (19).
Importantly, the cellular catalytic activity of BFT appears to be specific for E-cadherin, with no activity demonstrable on other cytoskeletal proteins, including the extracellular proteins occludin and β1-integrin and the intracellular proteins actin (42), ZO1 (associated with the cytoplasmic domain of occludin; ref. 43), or α- and β-catenins (associated with the cytoplasmic domain of E-cadherin; refs. 36, 37). These results are reminiscent of the striking substrate selectivity reported for the biologically important zinc-dependent metalloprotease enzymes tetanus toxin and botulinum toxin (39, 40). The specificity of BFT’s cellular proteolytic activity contrasts with the nonselective in vitro activity previously reported for BFT, including the proteolysis of actin (13). These discrepant data highlight the importance of assessing the activity of bacterial toxins by using pathogenically relevant experimental approaches. Furthermore, although E-cadherin is a well-known substrate of the nonspecific protease trypsin, treatment of HT29/C1 cells with trypsin does not lead to the cellular morphologic changes observed after BFT treatment, and the time course and proteolytic E-cadherin fragments generated by trypsin treatment are distinct from that observed with BFT (data not shown). For example, trypsin releases HT29/C1 cells from plastic, whereas BFT releases, at best, only a few HT29/C1 cells, consistent with its inability to degrade β1-integrin (Fig. 2).
Our data conflict with reports of Obiso et al. (7), who employed polarized MDCK cells and found that ZO1 staining diminished after BFT treatment, as assessed by standard fluorescent microscopy. These data were interpreted as suggesting that BFT cleavage of ZO1 may be causal in the reduced epithelial-monolayer resistance observed. However, our data using the complementary techniques of confocal immunofluorescent microscopy and Western blot analysis indicate that the ZO1 content of HT29/C1 cells is unchanged after BFT treatment, but that the cellular location of ZO1 is altered. The higher sensitivity and resolution of confocal microscopy explains, in part, the discrepant results. Nonetheless, the relocalization of ZO1 is likely to contribute to the reduced epithelial-monolayer resistance observed after BFT treatment. Our observation that BFT specifically cleaves E-cadherin, a protein essential to epithelial polarity and whose loss is associated with enhanced cellular metastatic potential (28), indicates that BFT is a potent cell biology tool to further study the complex protein–protein interactions present in epithelial cells. Studies to understand the subsequent steps in BFT’s mechanism of action should be particularly instructive in light of recent data suggesting that cell-surface proteolytic events (including proteolysis of the extracellular domain of tumor-suppressor molecules such as E-cadherin) trigger signaling functions that differ from those of the intact protein (45). Thus, further studies to characterize the signal transduction events and cytoskeletal changes triggered by BFT should enhance our understanding of cellular function, including the regulation of specific ion transporters by actin, and may yield insights permitting the development of novel therapeutic approaches to diarrheal disease. Our observations indicate that ETBF can be added to the growing list of bacteria and/or their toxins that can be utilized to investigate the biology and physiology of human polarized epithelial cells (19, 21, 41, 46, 47).
Acknowledgments
We thank Dr. James Nelson and his laboratory (particularly Kathy Siemers and Tzuu-Shuh Jou) for insightful discussions and for generously providing reagents for these experiments, Dwight Derr for tissue culture assistance, Dr. Shaoyu Chu for confocal microscopy assistance, and Drs. Mark Donowitz, Erik Hewlett, James Nataro, and Pat Morin for review of the manuscript. This work was supported by National Institutes of Health Award DK45496 to C.L.S.
ABBREVIATIONS
- CCCP
carbonyl cyanide m-chlorophenylhydrazone
- GAPDH
glyceraldehyde-3-phosphate dehydrogenase
References
- 1.Polk F B, Kasper D L. Ann Intern Med. 1996;86:569–571. doi: 10.7326/0003-4819-86-5-569. [DOI] [PubMed] [Google Scholar]
- 2.Tzianabos A O, Onderdonk A B, Rosner B, Cisneros R L, Kasper D L. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 3.Mundy L M, Sears C L. Clin Infect Dis. 1996;23:269–276. doi: 10.1093/clinids/23.2.269. [DOI] [PubMed] [Google Scholar]
- 4.Sears, C. L., Myers, L. L., Lazenby, A. & Van Tassell, R. L. (1995) Clin. Infect. Dis. 20, Suppl. 2, S142–S148. [DOI] [PubMed]
- 5.Myers L L, Shoop D S, Stackhouse L L, Newman F S, Flaherty R J, Letson G W, Sack R B. J Clin Microbiol. 1987;25:2330–2333. doi: 10.1128/jcm.25.12.2330-2333.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.San Joaquin V H, Griffis J C, Lee C, Sears C L. Scand J Infect Dis. 1995;27:211–215. doi: 10.3109/00365549509019011. [DOI] [PubMed] [Google Scholar]
- 7.Obiso R J, Jr, Azghani A O, Wilkins T D. Infect Immun. 1997;65:1431–1439. doi: 10.1128/iai.65.4.1431-1439.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Myers L L, Firehammer B D, Shoop D S, Border M M. Infect Immun. 1984;44:241–244. doi: 10.1128/iai.44.2.241-244.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Myers L L, Weikel C S. In: Enterotoxin as a Virulence Factor in Bacteroides fragilis-Associated Diarrhoeal Disease. Duerden B I, Brazier J S, Seddon S V, Wade W G, editors. Petersfield, U.K.: Wrightson Biomedical Publishing; 1992. pp. 90–100. [Google Scholar]
- 10.Sack R B, Myers L L, Almeido-Hill J, Shoop D S, Bradbury W C, Reid R, Santosham M. J Diarrhoeal Dis Res. 1992;10:4–9. [PubMed] [Google Scholar]
- 11.Sack R B, Albert M J, Alam K, Neogi P K B, Akbar M S. J Clin Microbiol. 1994;32:960–963. doi: 10.1128/jcm.32.4.960-963.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Van Tassell R L, Lyerly D M, Wilkins T D. Infect Immun. 1992;60:1343–1350. doi: 10.1128/iai.60.4.1343-1350.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moncrief J S, Obiso R, Barroso L A, Kling J J, Wright R L, Van Tassell R L, Lyerly D M, Wilkins T D. Infect Immun. 1995;63:175–181. doi: 10.1128/iai.63.1.175-181.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Franco A A, Mundy L M, Trucksis M, Wu S, Kaper J B, Sears C L. Infect Immun. 1997;65:1007–1013. doi: 10.1128/iai.65.3.1007-1013.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kling J J, Wright R L, Moncrief J S, Wilkens T D. FEMS Microbiol Lett. 1997;146:279–284. doi: 10.1016/s0378-1097(96)00488-0. [DOI] [PubMed] [Google Scholar]
- 16.Chambers F G, Koshy S S, Saidi R F, Clark D P, Moore R D, Sears C L. Infect Immun. 1997;65:3561–3570. doi: 10.1128/iai.65.9.3561-3570.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wells C L, Van De Westerlo E M A, Jechorek R P, Feltis B A, Wilkins T D, Erlandsen S L. Gastroenterology. 1996;110:1429–1437. doi: 10.1053/gast.1996.v110.pm8613048. [DOI] [PubMed] [Google Scholar]
- 18.Koshy S S, Montrose M H, Sears C L. Infect Immun. 1996;64:5022–5028. doi: 10.1128/iai.64.12.5022-5028.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mengaud J, Ohayon H, Gounon P, Mege R, Cossart P. Cell. 1996;84:923–932. doi: 10.1016/s0092-8674(00)81070-3. [DOI] [PubMed] [Google Scholar]
- 20.McCormick B A, Nusrat A, Parkos C A, D’Andrea L, Hofman P M, Carnes D, Liang T W, Madara J L. Infect Immun. 1997;65:1414–1421. doi: 10.1128/iai.65.4.1414-1421.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finlay B B, Cossart P. Science. 1997;276:718–725. doi: 10.1126/science.276.5313.718. [DOI] [PubMed] [Google Scholar]
- 22.Gumbiner B M. Cell. 1996;84:345–357. doi: 10.1016/s0092-8674(00)81279-9. [DOI] [PubMed] [Google Scholar]
- 23.Drubin D G, Nelson W J. Cell. 1996;84:335–344. doi: 10.1016/s0092-8674(00)81278-7. [DOI] [PubMed] [Google Scholar]
- 24.Fish E M, Molitoris B A. N Engl J Med. 1994;330:1580–1588. doi: 10.1056/NEJM199406023302207. [DOI] [PubMed] [Google Scholar]
- 25.Sebald M. Clin Infect Dis. 1994;18:S297–S304. doi: 10.1093/clinids/18.supplement_4.s297. [DOI] [PubMed] [Google Scholar]
- 26.Furuse M, Hirase T, Itoh M, Nagafuchi A, Yonemura S, Tsukita S. J Cell Biol. 1993;123:1777–1788. doi: 10.1083/jcb.123.6.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pouliot Y. BioEssays. 1992;14:743–748. doi: 10.1002/bies.950141104. [DOI] [PubMed] [Google Scholar]
- 28.Takeichi A. Science. 1991;251:1451–1455. doi: 10.1126/science.2006419. [DOI] [PubMed] [Google Scholar]
- 29.Saidi R F, Sears C L. Infect Immun. 1996;64:5029–5034. doi: 10.1128/iai.64.12.5029-5034.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Donelli G, Fabbri A, Fiorentini C. Infect Immun. 1996;64:113–119. doi: 10.1128/iai.64.1.113-119.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook J, Fritsch E F, Maniatis T. Molecular Cloning: A Laboratory Manual. 2nd Ed. Plainview, NY: Cold Spring Harbor Lab. Press; 1989. [Google Scholar]
- 32.Chen Y, Nelson W J. Anal Biochem. 1996;242:276–278. doi: 10.1006/abio.1996.0466. [DOI] [PubMed] [Google Scholar]
- 33.Weikel C S, Grieco F D, Reuben J, Myers L L, Sack R B. Infect Immun. 1992;60:321–327. doi: 10.1128/iai.60.2.321-327.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Marrs J A, Napolitano E W, Murphy-Erdosh C, Mays R A, Reichardt L F, Nelson W J. J Cell Biol. 1993;123:149–164. doi: 10.1083/jcb.123.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loktionova S A, Ilyinskaya O P, Gabai V L, Kabakov A E. FEBS Lett. 1996;392:100–104. doi: 10.1016/0014-5793(96)00792-2. [DOI] [PubMed] [Google Scholar]
- 36.Rimm D L, Koslov E R, Kebriaei P, Cianci C D, Morrow J S. Proc Natl Acad Sci USA. 1995;92:8813–8817. doi: 10.1073/pnas.92.19.8813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jou T, Stewart D B, Stappert J, Nelson W J, Marrs J A. Proc Natl Acad Sci USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Obiso R J, Jr, Lyerly D M, Van Tassell R L, Wilkins T D. Infect Immun. 1995;63:3820–3826. doi: 10.1128/iai.63.10.3820-3826.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tonello F, Montecucco C, Schiavo G, Rossetto O. Adv Exp Med Biol. 1996;389:251–260. [PubMed] [Google Scholar]
- 40.Williamson L C, Halpern J L, Montecucco C, Brown J E, Neale E A. J Biol Chem. 1996;271:7694–7699. doi: 10.1074/jbc.271.13.7694. [DOI] [PubMed] [Google Scholar]
- 41.Sears C L, Kaper J B. Microbiol Rev. 1996;60:167–215. doi: 10.1128/mr.60.1.167-215.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Saidi R F, Jaeger K, Montrose M H, Wu S, Sears C L. Cell Motil Cytoskeleton. 1997;37:159–165. doi: 10.1002/(SICI)1097-0169(1997)37:2<159::AID-CM8>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 43.Furuse M, Itoh M, Hirase T, Nagafuchi A, Yonemura S, Tsukita S. J Cell Biol. 1994;127:1617–1626. doi: 10.1083/jcb.127.6.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Anderson J M, Van Itallie C M. Am J Physiol. 1995;269:G467–G475. doi: 10.1152/ajpgi.1995.269.4.G467. [DOI] [PubMed] [Google Scholar]
- 45.Werb Z. Cell. 1997;91:439–442. doi: 10.1016/s0092-8674(00)80429-8. [DOI] [PubMed] [Google Scholar]
- 46.Cossart P, Boquet P, Normark S, Rappuoli R. Science. 1996;271:315–316. doi: 10.1126/science.271.5247.315. [DOI] [PubMed] [Google Scholar]
- 47.Finlay B B, Falkow S. Microbiol Mol Biol Rev. 1997;61:136–169. doi: 10.1128/mmbr.61.2.136-169.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]