Abstract
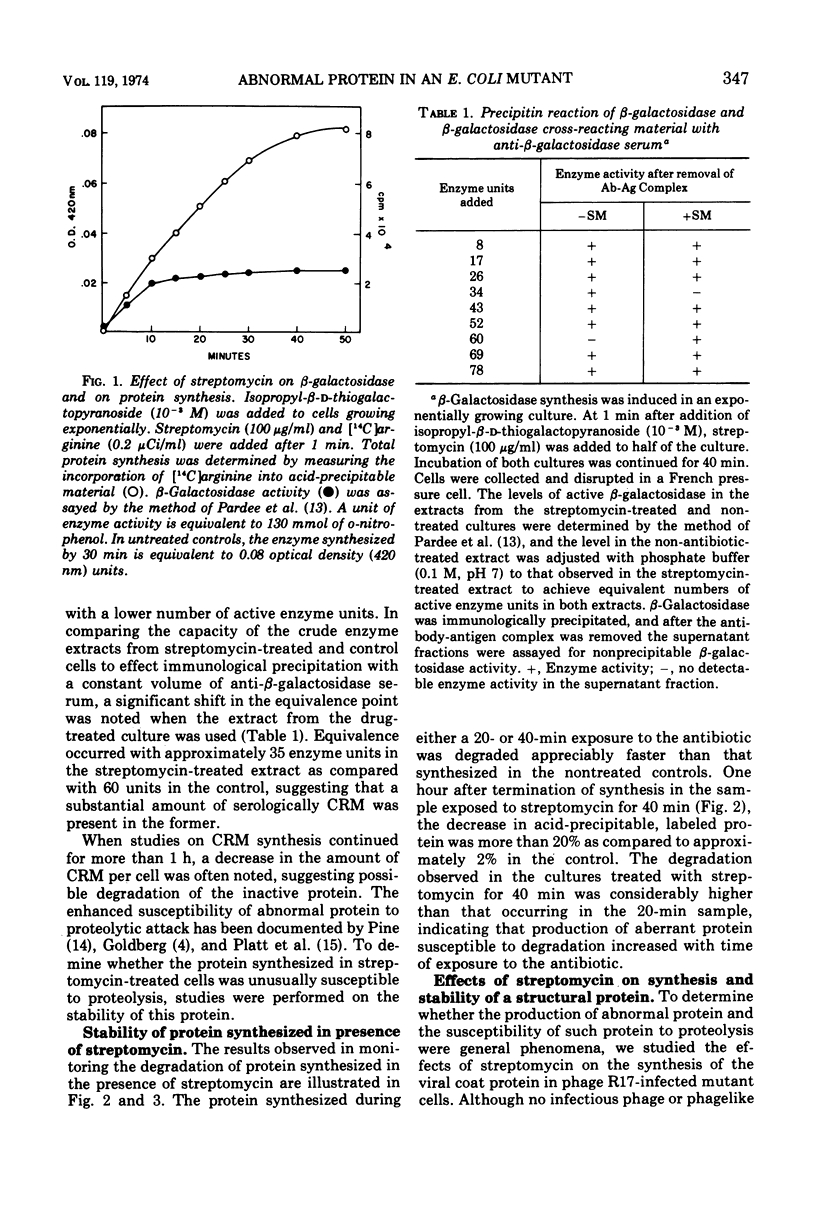
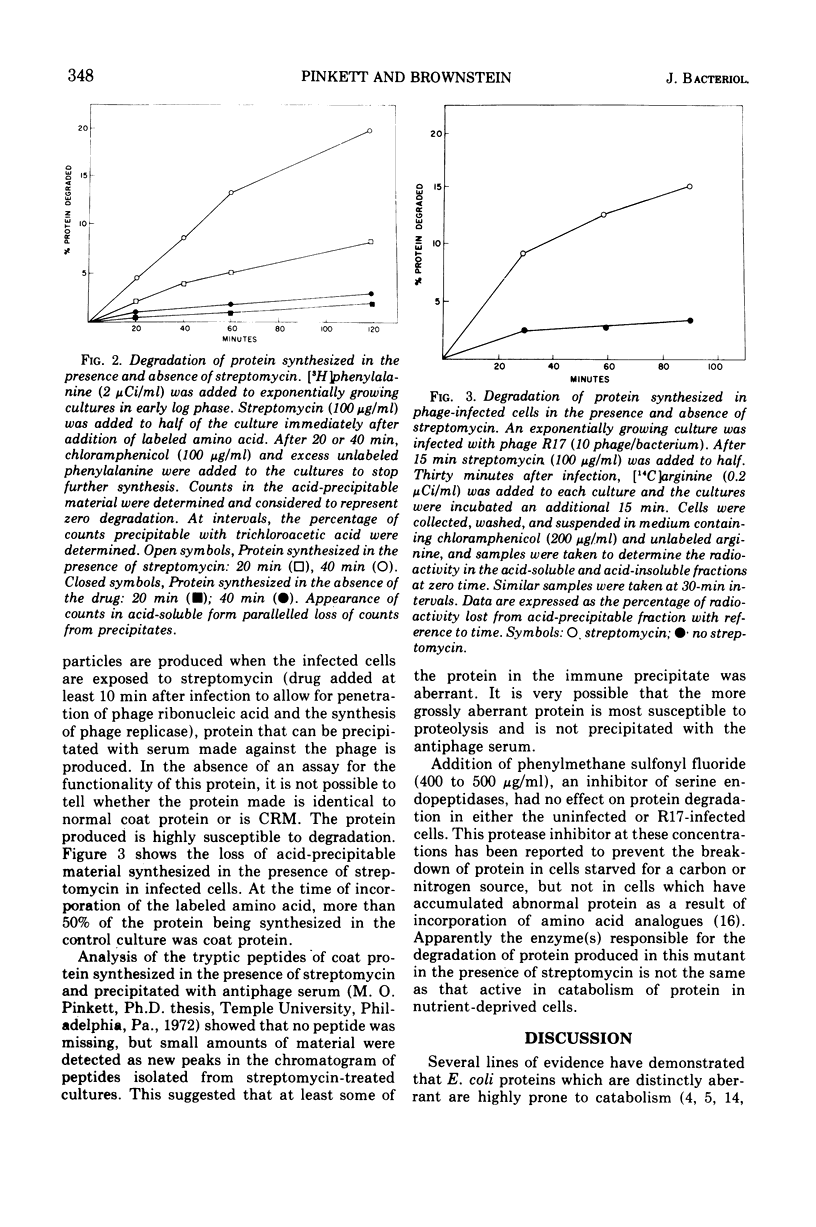
To determine directly the effects of streptomycin on translational fidelity in intact cells, we studied the synthesis of β-galactosidase and of the coat protein of bacteriophage R17 in an Escherichia coli mutant in which the bactericidal effects of streptomycin are delayed. After the addition of streptomycin to exponentially growing mutant cells, protein synthesis continues at an undiminished rate for approximately an hour; however, as measured by enzyme assays, little functional protein is produced. Serological assays designed to detect β-galactosidase and bacteriophage R17 coat protein show that substantial amounts of the protein synthesized can react with antisera prepared against active β-galactosidase and phage R17, indicating the aberrance of the protein produced in the presence of the antibiotic. The polypeptides synthesized in the presence of streptomycin are degraded in the cell to a much greater extent than protein synthesized in the absence of the antibiotic. The proteolytic attack on this protein is not affected by inhibitors of serine proteases, suggesting that enzymes other than those involved in “normal turnover” of cellular protein are responsible. In this strain, certain of the multiple effects of streptomycin are separated in time and the production of abnormal protein (enzymatically inactive and susceptible to proteolytic attack) could be studied in the absence of the lethal effect of the drug.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atkins J. F., Elseviers D., Gorini L. Low activity of -galactosidase in frameshift mutants of Escherichia coli. Proc Natl Acad Sci U S A. 1972 May;69(5):1192–1195. doi: 10.1073/pnas.69.5.1192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissell D. M. Formation of an altered enzyme by Escherichia coli in the presence of neomycin. J Mol Biol. 1965 Dec;14(2):619–622. doi: 10.1016/s0022-2836(65)80215-7. [DOI] [PubMed] [Google Scholar]
- Brownstein B. L., Lewandowski L. J. A mutation suppressing streptomycin dependence. I. An effect on ribosome function. J Mol Biol. 1967 Apr 14;25(1):99–109. doi: 10.1016/0022-2836(67)90281-1. [DOI] [PubMed] [Google Scholar]
- GORINI L., KATAJA E. STREPTOMYCIN-INDUCED OVERSUPPRESSION IN E. COLI. Proc Natl Acad Sci U S A. 1964 Jun;51:995–1001. doi: 10.1073/pnas.51.6.995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A. L. Degradation of abnormal proteins in Escherichia coli (protein breakdown-protein structure-mistranslation-amino acid analogs-puromycin). Proc Natl Acad Sci U S A. 1972 Feb;69(2):422–426. doi: 10.1073/pnas.69.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt R. In vivo degradation of nonsense fragments in E. coli. Nature. 1970 Dec 19;228(5277):1151–1154. doi: 10.1038/2281151a0. [DOI] [PubMed] [Google Scholar]
- Gorini L., Beckwith J. R. Suppression. Annu Rev Microbiol. 1966;20:401–422. doi: 10.1146/annurev.mi.20.100166.002153. [DOI] [PubMed] [Google Scholar]
- Gorini L. The contrasting role of strA and ram gene products in ribosomal functioning. Cold Spring Harb Symp Quant Biol. 1969;34:101–109. doi: 10.1101/sqb.1969.034.01.016. [DOI] [PubMed] [Google Scholar]
- Kreider G., Brownstein B. L. A mutation suppressing streptomycin dependence. II. An altered protein on the 30 s ribosomal subunit. J Mol Biol. 1971 Oct 14;61(1):135–142. doi: 10.1016/0022-2836(71)90211-7. [DOI] [PubMed] [Google Scholar]
- Modolell J., Davis B. D. Breakdown by streptomycin of initiation complexes formed on ribosomes of Escherichia coli. Proc Natl Acad Sci U S A. 1970 Nov;67(3):1148–1155. doi: 10.1073/pnas.67.3.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKADA D., MAGASANIK B. THE ROLES OF INDUCER AND CATABOLITE REPRESSOR IN THE SYNTHESIS OF BETA-GALACTOSIDASE BY ESCHERICHIA COLI. J Mol Biol. 1964 Jan;8:105–127. doi: 10.1016/s0022-2836(64)80153-4. [DOI] [PubMed] [Google Scholar]
- PARANCHYCH W., GRAHAM A. F. Isolation and properties of an RNA-containing bacteriophage. J Cell Comp Physiol. 1962 Dec;60:199–208. doi: 10.1002/jcp.1030600303. [DOI] [PubMed] [Google Scholar]
- Pine M. J. Response of intracellular proteolysis to alteration of bacterial protein and the implications in metabolic regulation. J Bacteriol. 1967 May;93(5):1527–1533. doi: 10.1128/jb.93.5.1527-1533.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt T., Miller J. H., Weber K. In vivo degradation of mutant lac repressor. Nature. 1970 Dec 19;228(5277):1154–1156. doi: 10.1038/2281154a0. [DOI] [PubMed] [Google Scholar]
- Prouty W. F., Goldberg A. L. Effects of protease inhibitors on protein breakdown in Escherichia coli. J Biol Chem. 1972 May 25;247(10):3341–3352. [PubMed] [Google Scholar]
- Prouty W. F., Goldberg A. L. Fate of abnormal proteins in E. coli accumulation in intracellular granules before catabolism. Nat New Biol. 1972 Nov 29;240(100):147–150. doi: 10.1038/newbio240147a0. [DOI] [PubMed] [Google Scholar]
- Weisblum B., Davies J. Antibiotic inhibitors of the bacterial ribosome. Bacteriol Rev. 1968 Dec;32(4 Pt 2):493–528. [PMC free article] [PubMed] [Google Scholar]
- Zimmermann R. A., Garvin R. T., Gorini L. Alteration of a 30S ribosomal protein accompanying the ram mutation in Escherichia coli. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2263–2267. doi: 10.1073/pnas.68.9.2263. [DOI] [PMC free article] [PubMed] [Google Scholar]


