Abstract
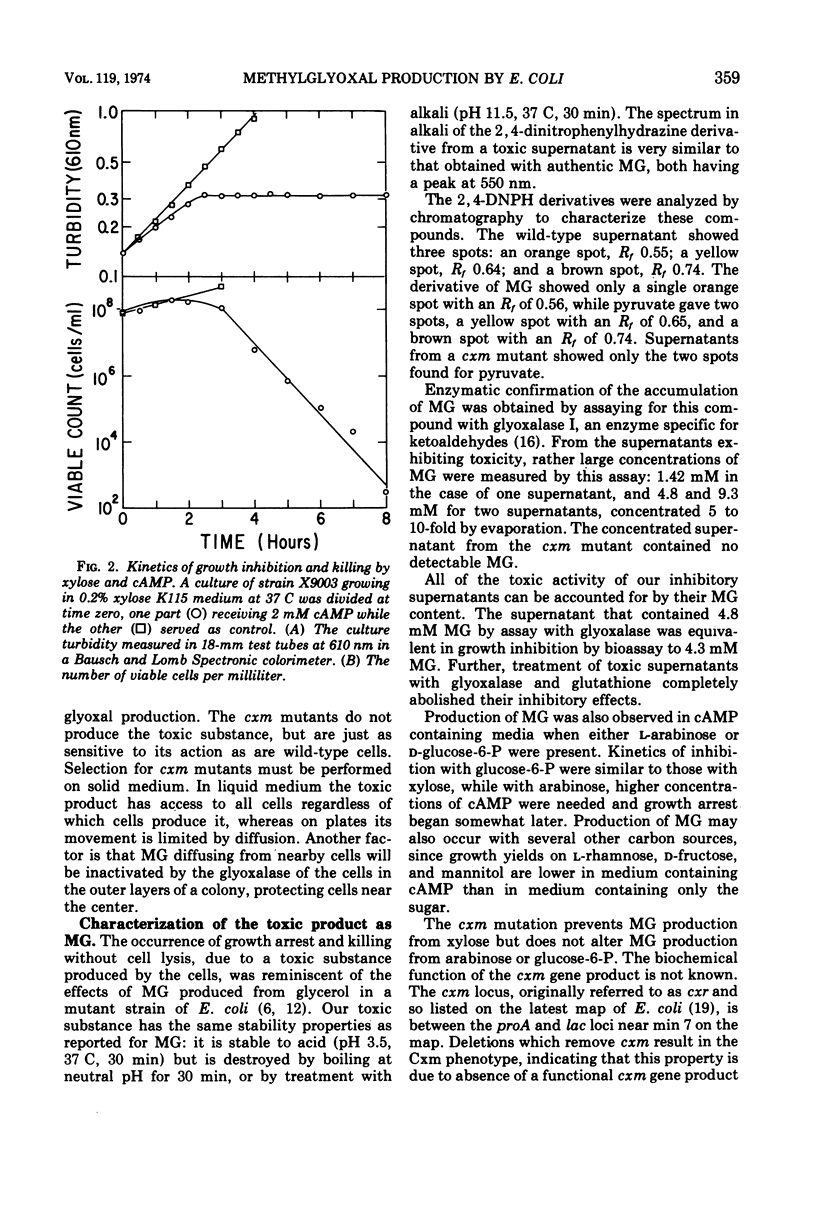
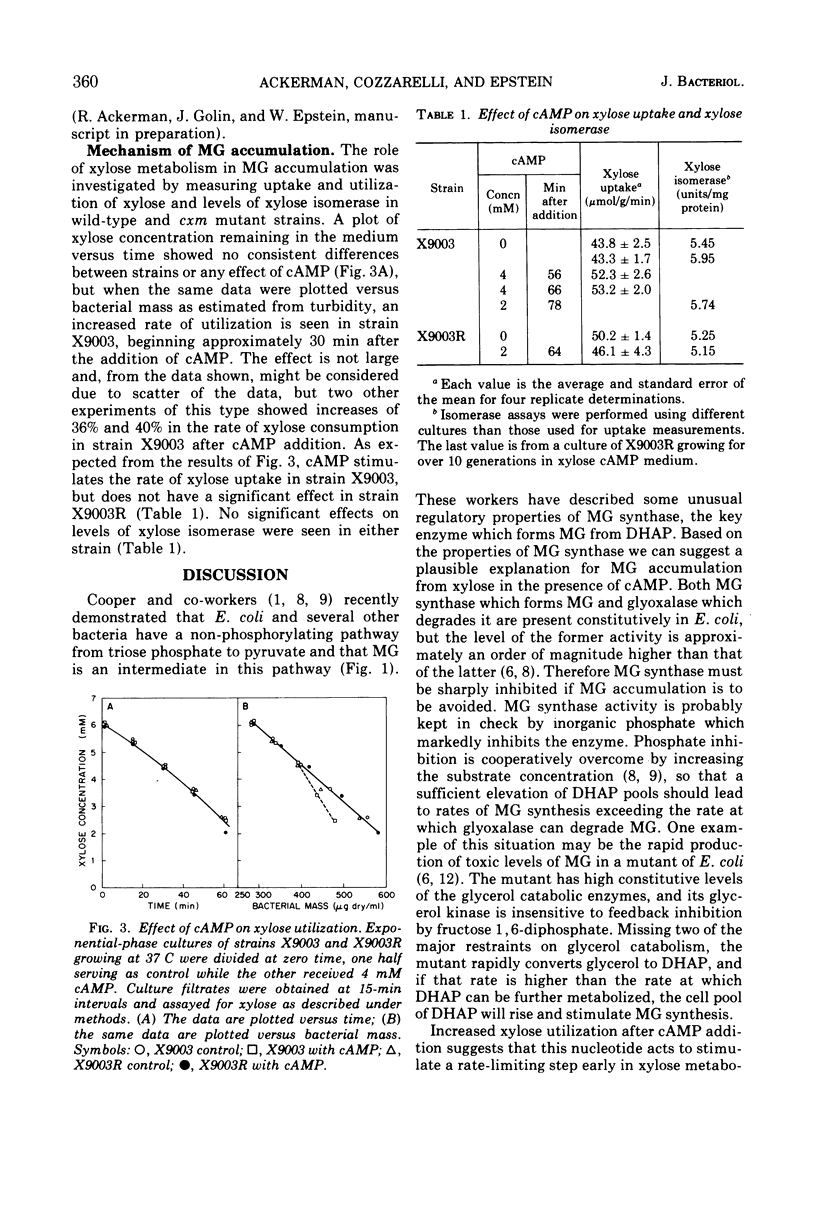
Wild-type strains of Escherichia coli K-12 accumulate toxic concentrations of methylglyoxal when grown in medium containing adenosine 3′,5′-monophosphate and either d-xylose, l-arabinose, or d-glucose-6-phosphate, independent of the presence of other carbon sources. Mutations at a locus called cxm specifically block methylglyoxal formation from xylose in the presence of adenosine 3′,5′-monophosphate. Accumulation in medium containing xylose, studied in some detail, is dependent on the ability to utilize xylose and is associated with an increased rate of xylose utilization without changes in levels of xylose isomerase. These results suggest that adenosine 3′,5′-monophosphate results in induction of excessively high levels of an early rate-limiting step in xylose metabolism. This step may be the transport of xylose into the cell. The resulting excessive rates of xylose catabolism could stimulate methylglyoxal formation by overburdening late steps in glycolysis.
Full text
PDF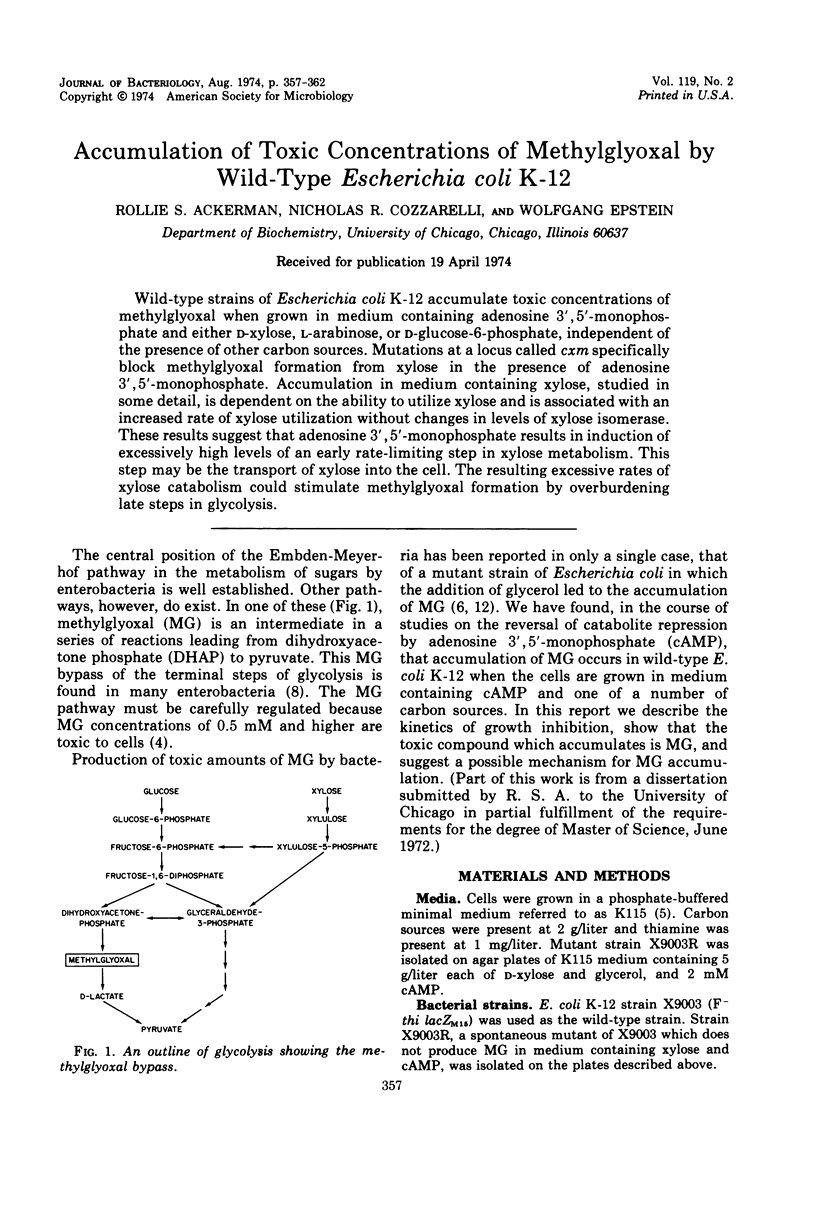



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Cooper R. A., Anderson A. The formation and catabolism of methylglyoxal during glycolysis in Escherichia coli. FEBS Lett. 1970 Dec 11;11(4):273–276. doi: 10.1016/0014-5793(70)80546-4. [DOI] [PubMed] [Google Scholar]
- DISCHE Z., BORENFREUND E. A new spectrophotometric method for the detection and determination of keto sugars and trioses. J Biol Chem. 1951 Oct;192(2):583–587. [PubMed] [Google Scholar]
- David J. D., Wiesmeyer H. Control of xylose metabolism in Escherichia coli. Biochim Biophys Acta. 1970 Mar 24;201(3):497–499. doi: 10.1016/0304-4165(70)90171-6. [DOI] [PubMed] [Google Scholar]
- Együd L. G., Szent-Györgyi A. Cell division, SH, ketoaldehydes, and cancer. Proc Natl Acad Sci U S A. 1966 Feb;55(2):388–393. doi: 10.1073/pnas.55.2.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein W., Kim B. S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971 Nov;108(2):639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedberg W. B., Kistler W. S., Lin E. C. Lethal synthesis of methylglyoxal by Escherichia coli during unregulated glycerol metabolism. J Bacteriol. 1971 Oct;108(1):137–144. doi: 10.1128/jb.108.1.137-144.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Cooper R. A. The purification and properties of Escherichia coli methylglyoxal synthase. Biochem J. 1972 Jun;128(2):321–329. doi: 10.1042/bj1280321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper D. J., Cooper R. A. The regulation of Escherichia coli methylglyoxal synthase; a new control site in glycolysis? FEBS Lett. 1971 Mar 16;13(4):213–216. doi: 10.1016/0014-5793(71)80538-0. [DOI] [PubMed] [Google Scholar]
- Katz L., Englesberg E. Hyperinducibility as a result of mutation in structural genes and self-catabolite repression in the ara operon. J Bacteriol. 1971 Jul;107(1):34–52. doi: 10.1128/jb.107.1.34-52.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krymkiewicz N., Diéguez E., Rekarte U. D., Zwaig N. Properties and mode of action of a bactericidal compound (=methylglyoxal) produced by a mutant of Escherichia coli. J Bacteriol. 1971 Dec;108(3):1338–1347. doi: 10.1128/jb.108.3.1338-1347.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Pastan I., Perlman R. Cyclic adenosine monophosphate in bacteria. Science. 1970 Jul 24;169(3943):339–344. doi: 10.1126/science.169.3943.339. [DOI] [PubMed] [Google Scholar]
- Rothman-Denes L. B., Hesse J. E., Epstein W. Role of cyclic adenosine 3',5'-monophosphate in the in vivo expression of the galactose operon of Escherichia coli. J Bacteriol. 1973 Jun;114(3):1040–1044. doi: 10.1128/jb.114.3.1040-1044.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Setlow P. Inability of detect cyclic AMP in vegetative or sporulating cells or dormant spores of Bacillus megaterium. Biochem Biophys Res Commun. 1973 May 15;52(2):365–372. doi: 10.1016/0006-291x(73)90720-1. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]


