Abstract
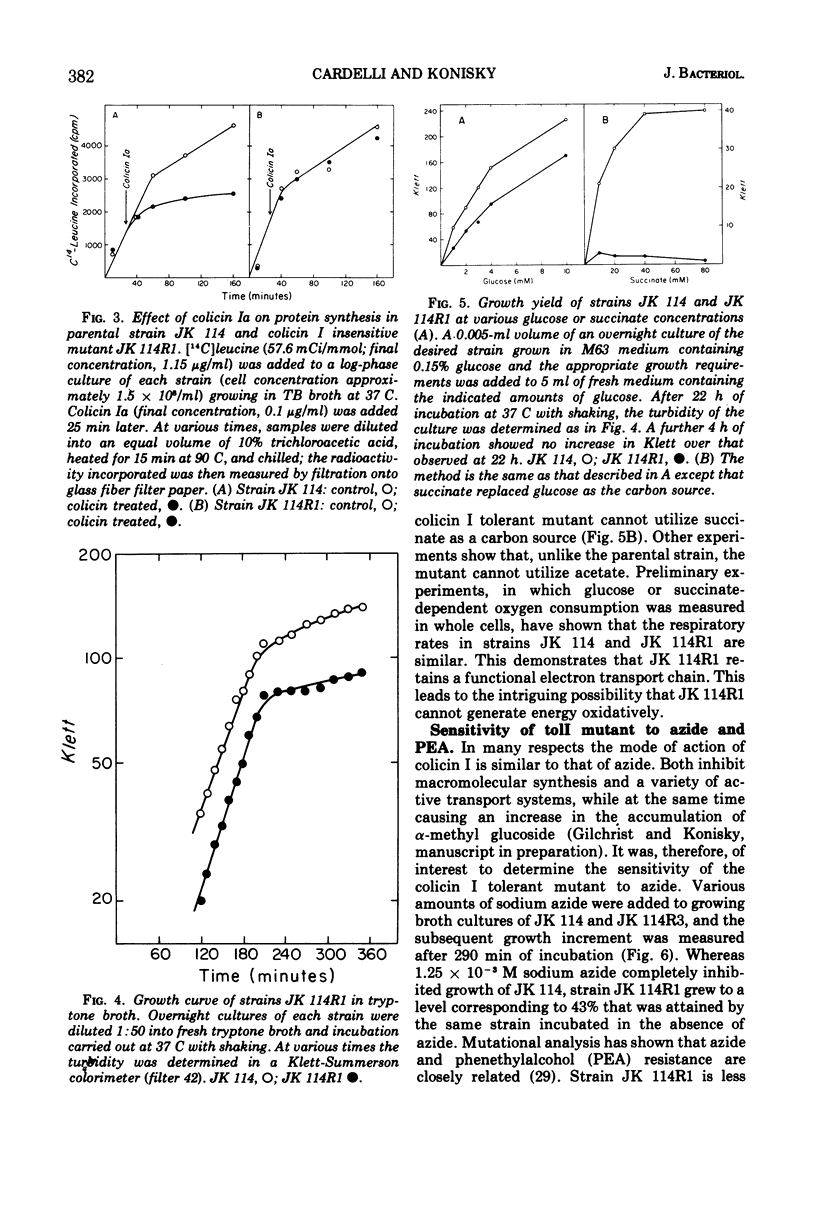
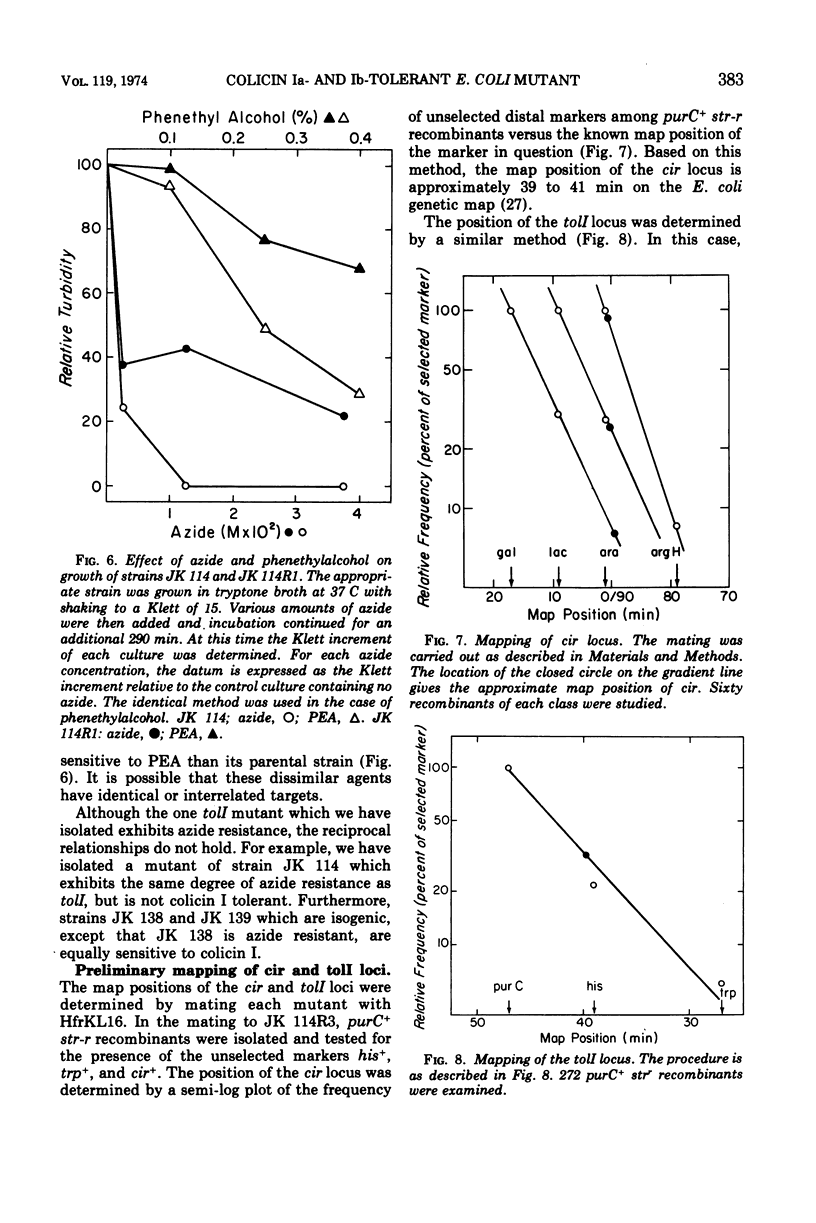
Two classes of spontaneous colicin I insensitive mutants of Escherichia coli have been isolated. The first class (called cir) has lost its ability to adsorb either colicin Ia or Ib, maps at 41 min on the E. coli genetic map, and retains sensitivity to all other colicins tested. The cir phenotype is probably due to an alteration in the colicin I receptor. The second class of mutant (called tolI) retains full capacity to adsorb [125I]colicin I and, therefore, represents the isolation of a mutant tolerant to colicin I. The tolI mutant is sensitive to all other colicins tested and has a map location of 89-1 min. The tolI mutant grows with a reduced mass yield when glucose is used as a carbon source and cannot utilize succinate or acetate for growth. The tolI mutant shows a reduced sensitivity to sodium azide and phenethylalcohol. It is suggested that tolI is deficient in some aspect of aerobic metabolism which must be operative for colicin I sensitivity.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bernstein A., Rolfe B., Onodera K. Pleiotropic properties and genetic organization of the tolA,B locus of Escherichia coli K-12. J Bacteriol. 1972 Oct;112(1):74–83. doi: 10.1128/jb.112.1.74-83.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Wolff H. Characterization of the receptor protein for phage T5 and colicin M in the outer membrane of E. coli B. FEBS Lett. 1973 Aug 1;34(1):77–80. doi: 10.1016/0014-5793(73)80707-0. [DOI] [PubMed] [Google Scholar]
- Burman L. G., Nordström K. Colicin tolerance induced by ampicillin or mutation to ampicillin resistance in a strain of Escherichia coli K-12. J Bacteriol. 1971 Apr;106(1):1–13. doi: 10.1128/jb.106.1.1-13.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K-12: the genetic and biochemical characterisations of a strain carrying a mutation in the uncB gene. Biochim Biophys Acta. 1973 Feb 22;292(2):366–375. doi: 10.1016/0005-2728(73)90043-1. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Haan P. G., Hoekstra W. P., Verhoef C., Felix H. S. Recombination in Escherichia coli. 3. Mapping by the gradient of transmission. Mutat Res. 1969 Nov-Dec;8(3):505–512. doi: 10.1016/0027-5107(69)90067-0. [DOI] [PubMed] [Google Scholar]
- Eriksson-Grennberg K. G., Nordström K. Genetics and physiology of a tolE mutant of Escherichia coli K-12 and phenotypic suppression of its phenotype by galactose. J Bacteriol. 1973 Sep;115(3):1219–1222. doi: 10.1128/jb.115.3.1219-1222.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on cellular metabolism. J Bacteriol. 1969 Jan;97(1):64–77. doi: 10.1128/jb.97.1.64-77.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fields K. L., Luria S. E. Effects of colicins E1 and K on transport systems. J Bacteriol. 1969 Jan;97(1):57–63. doi: 10.1128/jb.97.1.57-63.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J., Barrett C. Characterization of Escherichia coli mutants tolerant to bacteriocin JF246: two new classes of tolerant mutants. J Bacteriol. 1973 Nov;116(2):885–892. doi: 10.1128/jb.116.2.885-892.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foulds J. Mode of action of a bacteriocin from Serratia marcescens. J Bacteriol. 1971 Sep;107(3):833–839. doi: 10.1128/jb.107.3.833-839.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K. Colicin B: mode of action and inhibition by enterochelin. J Bacteriol. 1973 Jun;114(3):1217–1224. doi: 10.1128/jb.114.3.1217-1224.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guterman S. K., Luria S. E. Escherichia coli: strains that excrete an inhibitor of colicin B. Science. 1969 Jun 20;164(3886):1414–1414. doi: 10.1126/science.164.3886.1414. [DOI] [PubMed] [Google Scholar]
- Konisky J. Characterization of colicin Ia and colicin Ib. Chemical studies of protein structure. J Biol Chem. 1972 Jun 25;247(12):3750–3755. [PubMed] [Google Scholar]
- Konisky J., Cowell B. S., Gilchrist M. J. Colicin Ia and Ib binding to Escherichia coli envelopes and partially purified cell walls. J Supramol Struct. 1973;1(3):208–219. doi: 10.1002/jss.400010306. [DOI] [PubMed] [Google Scholar]
- Konisky J., Cowell B. S. Interaction of colicin Ia with bacterial cells. Direct measurement of Ia-receptor interaction. J Biol Chem. 1972 Oct 25;247(20):6524–6529. [PubMed] [Google Scholar]
- Konisky J., Liu C. T. Solubilization and partial characterization of the colicin I receptor of Escherichia coli. J Biol Chem. 1974 Feb 10;249(3):835–840. [PubMed] [Google Scholar]
- Levisohn R., Konisky J., Nomura M. Interaction of colicins with bacterial cells. IV. Immunity breakdown studied with colicins Ia and Ib. J Bacteriol. 1968 Sep;96(3):811–821. doi: 10.1128/jb.96.3.811-821.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NOMURA M. MECHANISM OF ACTION OF COLICINES. Proc Natl Acad Sci U S A. 1964 Dec;52:1514–1521. doi: 10.1073/pnas.52.6.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R., Luria S. E. Genetics and physiology of colicin-tolerant mutants of Escherichia coli. J Bacteriol. 1967 Oct;94(4):1112–1123. doi: 10.1128/jb.94.4.1112-1123.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagel de Zwaig R. Mode of action of colicin A. J Bacteriol. 1969 Sep;99(3):913–914. doi: 10.1128/jb.99.3.913-914.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nomura M., Witten C. Interaction of colicins with bacterial cells. 3. Colicin-tolerant mutations in Escherichia coli. J Bacteriol. 1967 Oct;94(4):1093–1111. doi: 10.1128/jb.94.4.1093-1111.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabet S. F., Schnaitman C. A. Localization and solubilization of colicin receptors. J Bacteriol. 1971 Oct;108(1):422–430. doi: 10.1128/jb.108.1.422-430.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Threlfall E. J., Holland I. B. Co-transduction with serB of a pleiotropic mutation affecting colicin E2 refractivity, ultraviolet sensitivity, recombination proficiency and surface properties of Escherichia coli K12. J Gen Microbiol. 1970 Aug;62(3):383–398. doi: 10.1099/00221287-62-3-383. [DOI] [PubMed] [Google Scholar]
- Whitney E. N. The tolC locus in Escherichia coli K12. Genetics. 1971 Jan;67(1):39–53. doi: 10.1093/genetics/67.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yura T., Wada C. Phenethyl alcohol resistance in Escherichia coli. I. Resistance of strain C600 and its relation to azide resistance. Genetics. 1968 Jun;59(2):177–190. doi: 10.1093/genetics/59.2.177. [DOI] [PMC free article] [PubMed] [Google Scholar]


