Abstract
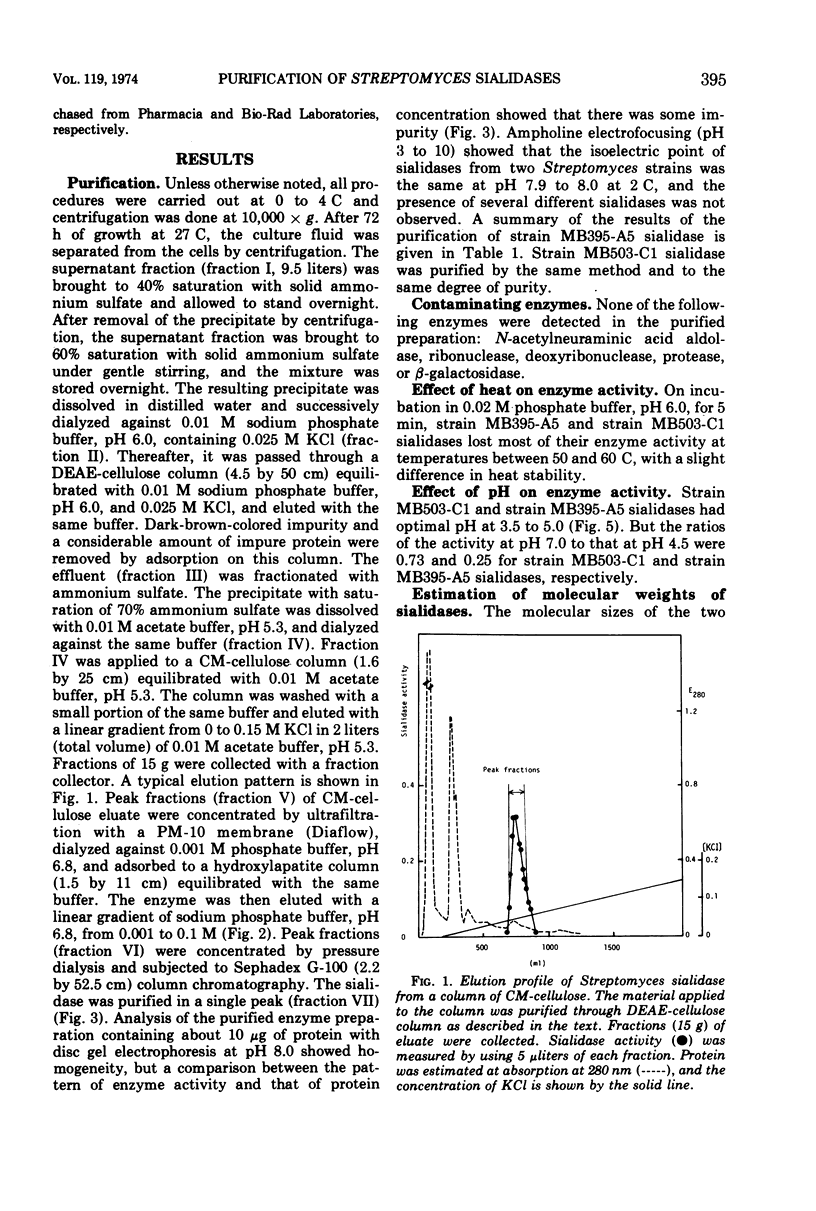
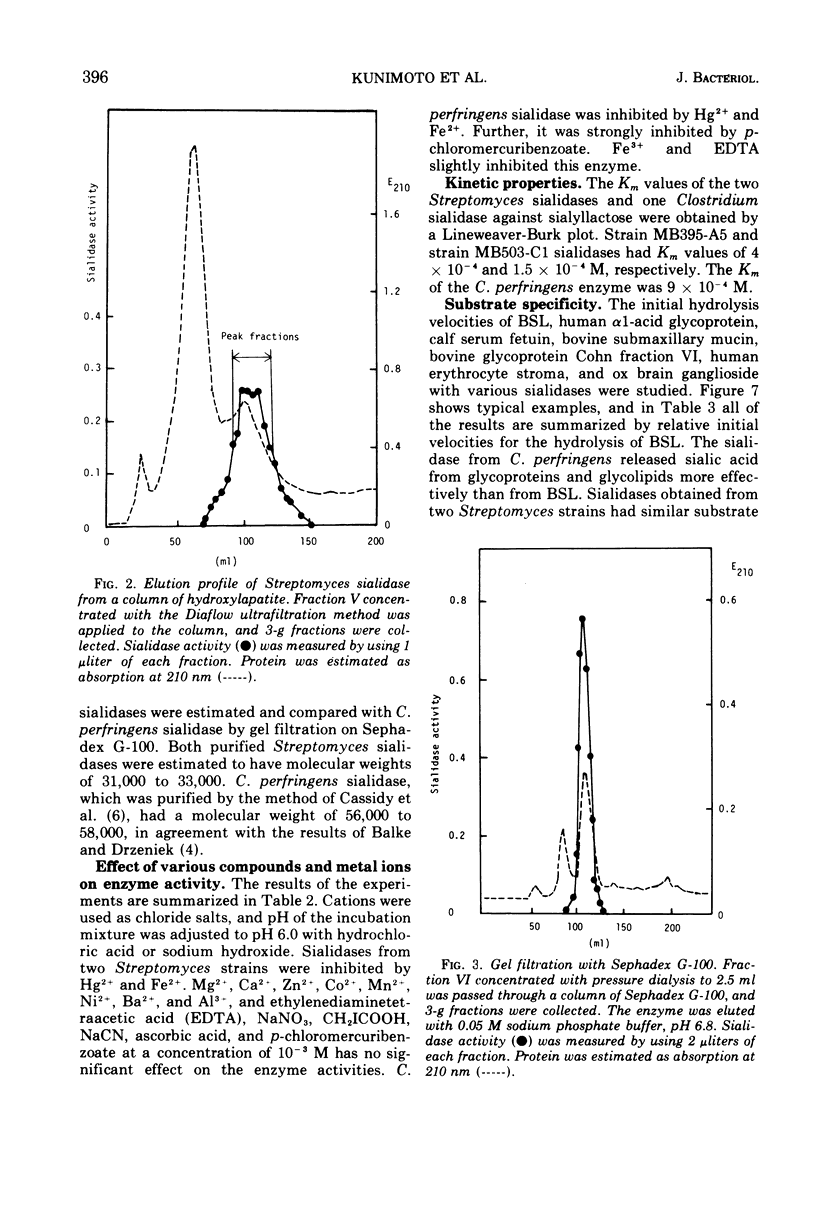
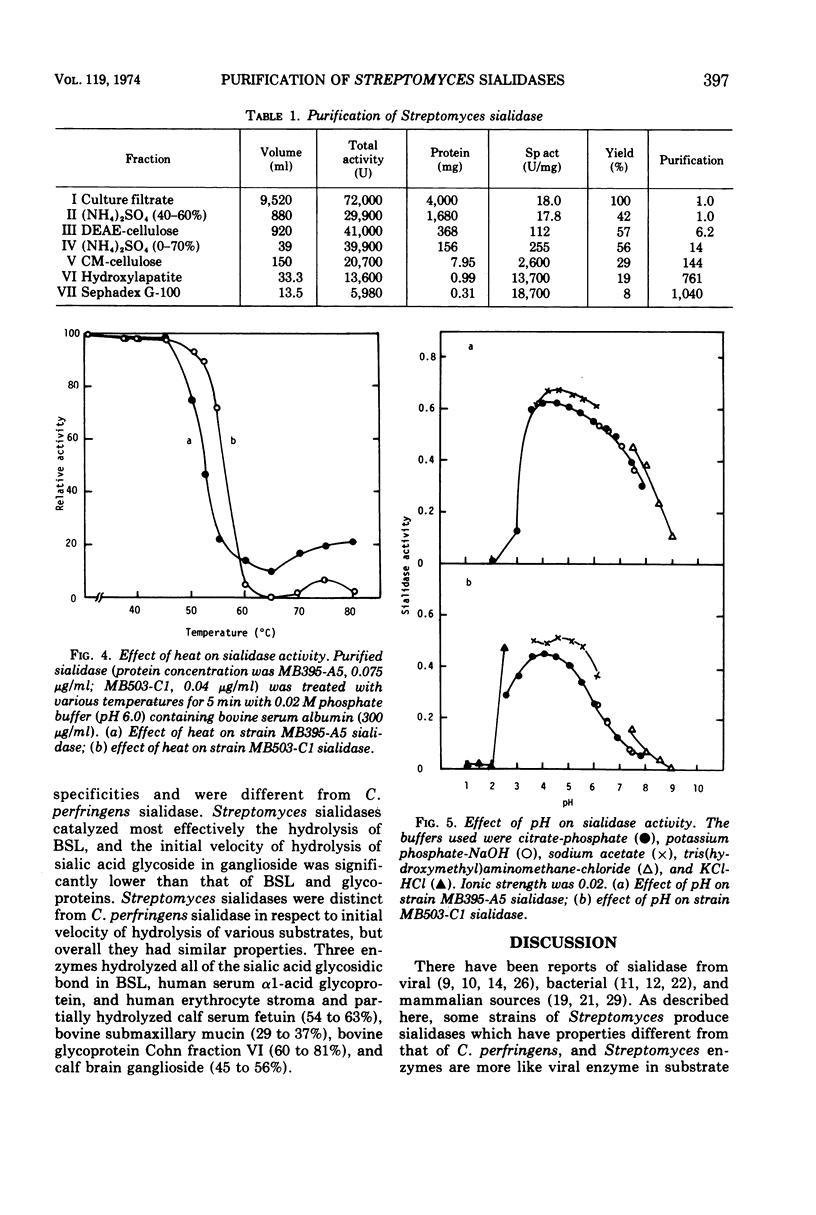
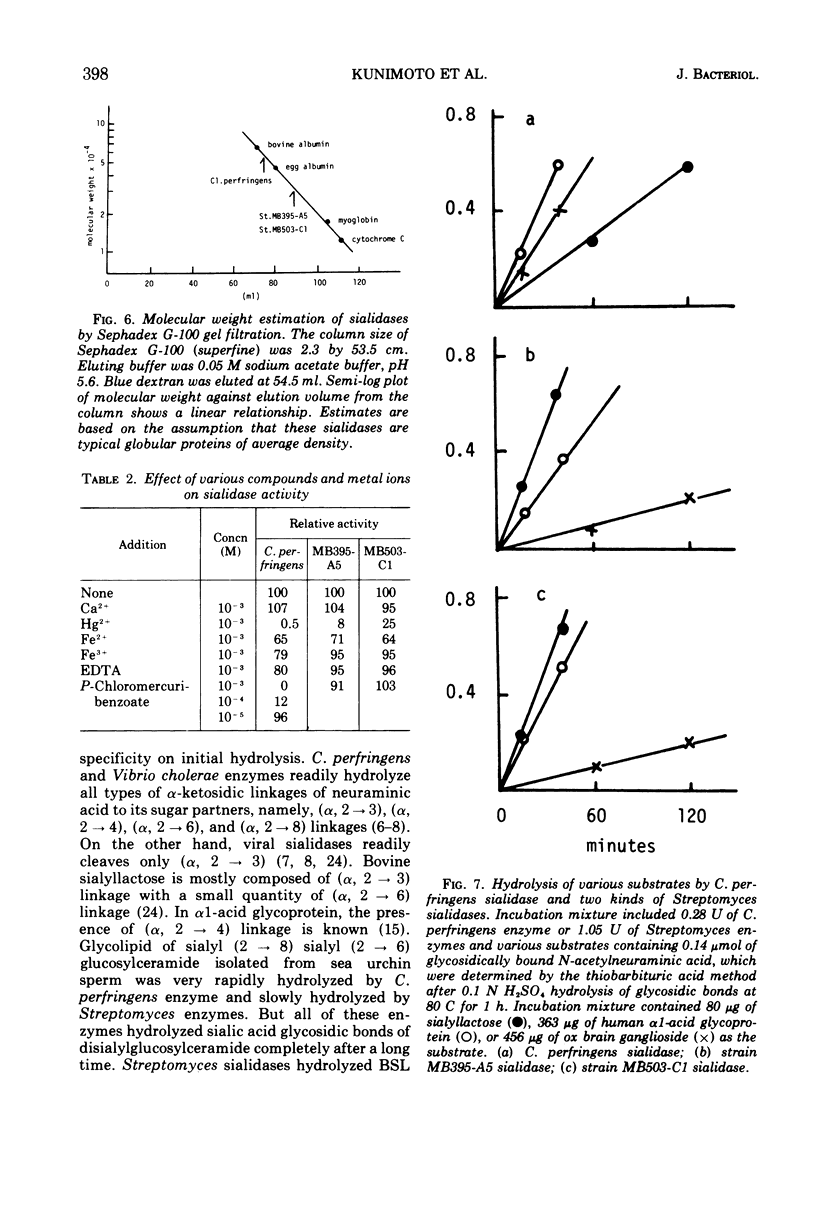
Some strains of Streptomyces produce sialidases. Two sialidases were purified over 1,000-fold from a culture filtrate of two Streptomyces species. They had the same properties in molecular weight, behavior to ions and other reagents, and substrate specificity. They showed very small differences in kinetic properties, pH optima, and heat stability. These Streptomyces sialidases differed markedly from Clostridium perfringens sialidase in molecular weight, p-chloromercuribenzoate sensitivity, and substrate specificity. Approximate molecular weights of the sialidases from Streptomyces and C. perfringens were 32,000 and 57,000, respectively. p-Chloromercuribenzoate (10−3 M) caused complete inhibition of C. perfringens sialidase but not of Streptomyces sialidases.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADA G. L., FRENCH E. L., LIND P. E. Purification and properties of neuraminidase from Vibrio cholerae. J Gen Microbiol. 1961 Mar;24:409–425. doi: 10.1099/00221287-24-3-409. [DOI] [PubMed] [Google Scholar]
- ADA G. L., FRENCH E. L. Purification of bacterial neuraminidase (receptor-destroying enzyme). Nature. 1959 Jun 20;183(4677):1740–1741. doi: 10.1038/1831740b0. [DOI] [PubMed] [Google Scholar]
- Aoyagi T., Yagisawa M., Kumagai M., Hamada M., Okami Y. An enzyme inhibitor, panosialin, produced by Streptomyces. I. Biological activity, isolation and characterization of panosialin. J Antibiot (Tokyo) 1971 Dec;24(12):860–869. doi: 10.7164/antibiotics.24.860. [DOI] [PubMed] [Google Scholar]
- Balke E., Drzeniek R. Untersuchungen über die Clostridium perfringens-Neuraminidase. Z Naturforsch B. 1969 May;24(5):599–603. [PubMed] [Google Scholar]
- Braunstein G. D., Reichert L. E., Jr, Van Hall E. V., Vaitukaitis J. L., Ross G. T. The effects of desialylation on the biologic and immunologic activity of human pituitary luteinizing hormone. Biochem Biophys Res Commun. 1971 Mar 5;42(5):962–967. doi: 10.1016/0006-291x(71)90524-9. [DOI] [PubMed] [Google Scholar]
- Cassidy J. T., Jourdian G. W., Roseman S. The sialic acids. VI. Purification and properties of sialidase from Clostridium perfringens. J Biol Chem. 1965 Sep;240(9):3501–3506. [PubMed] [Google Scholar]
- Drzeniek R. Differences in splitting capacity of virus and V. cholerae neuraminidases on sialic acid type substrates. Biochem Biophys Res Commun. 1967 Mar 21;26(6):631–638. doi: 10.1016/s0006-291x(67)80118-9. [DOI] [PubMed] [Google Scholar]
- Drzeniek R., Gauhe A. Differences in substrate specificity of myxovirus neuraminidases. Biochem Biophys Res Commun. 1970 Feb 20;38(4):651–656. doi: 10.1016/0006-291x(70)90630-3. [DOI] [PubMed] [Google Scholar]
- GOTTSCHALK A. Neuraminidase: the specific enzyme of influenza virus and Vibrio cholerae. Biochim Biophys Acta. 1957 Mar;23(3):645–646. doi: 10.1016/0006-3002(57)90389-x. [DOI] [PubMed] [Google Scholar]
- Heimer R., Meyer K. STUDIES ON SIALIC ACID OF SUBMAXILLARY MUCOID. Proc Natl Acad Sci U S A. 1956 Oct;42(10):728–734. doi: 10.1073/pnas.42.10.728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotta K., Horikawa M., Kurokawa M. Purification and characterization of alpha 1-acid glycoprotein from ascitic fluid. Anal Biochem. 1970 Feb;33(2):463–468. doi: 10.1016/0003-2697(70)90317-9. [DOI] [PubMed] [Google Scholar]
- Howe C., Newcomb E. W., Lee L. T. The neuraminidase of measles virus. Biochem Biophys Res Commun. 1969 Feb 21;34(4):388–391. doi: 10.1016/0006-291x(69)90393-3. [DOI] [PubMed] [Google Scholar]
- JEANLOZ R. W. [Recent research on the chemistry of complex polyosides containing amino sugars]. Bull Soc Chim Biol (Paris) 1960;42:303–325. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Mahadevan S., Nduguba J. C., Tappel A. L. Sialidase of rat liver and kidney. J Biol Chem. 1967 Oct 10;242(19):4409–4413. [PubMed] [Google Scholar]
- Morell A. G., Gregoriadis G., Scheinberg I. H., Hickman J., Ashwell G. The role of sialic acid in determining the survival of glycoproteins in the circulation. J Biol Chem. 1971 Mar 10;246(5):1461–1467. [PubMed] [Google Scholar]
- Ohman R., Rosenberg A., Svennerholm L. Human brain sialidase. Biochemistry. 1970 Sep 15;9(19):3774–3782. doi: 10.1021/bi00821a017. [DOI] [PubMed] [Google Scholar]
- POPENOE E. A., DREW R. M. The action of an enzyme of Clostridium perfringens on orosomucoid. J Biol Chem. 1957 Oct;228(2):673–683. [PubMed] [Google Scholar]
- RAFELSON M. E., Jr, SCHNEIR M., WILSON V. W., Jr STUDIES ON THE NEURAMINIDASE OF INFLUENZA VIRUS. II. ADDITIONAL PROPERTIES OF THE ENZYMES FROM THE ASIAN AND PR 8 STRAINS. Arch Biochem Biophys. 1963 Dec;103:424–430. doi: 10.1016/0003-9861(63)90432-6. [DOI] [PubMed] [Google Scholar]
- SCHRAMM G., MOHR E. Purification of neuraminidase from Vibrio cholerae. Nature. 1959 Jun 13;183(4676):1677–1678. doi: 10.1038/1831677a0. [DOI] [PubMed] [Google Scholar]
- SETO J. T., HICKEY B. J., RASMUSSEN A. F., Jr Sialidase activity and related properties of influenza A2 viruses. Virology. 1959 Dec;9:598–611. doi: 10.1016/0042-6822(59)90151-5. [DOI] [PubMed] [Google Scholar]
- SPIRO R. G. PERIODATE OXIDATION OF THE GLYCOPROTEIN FETUIN. J Biol Chem. 1964 Feb;239:567–573. [PubMed] [Google Scholar]
- WARREN L., SPEARING C. W. Mammalian sialidase (neuraminidase). Biochem Biophys Res Commun. 1960 Nov;3:489–492. doi: 10.1016/0006-291x(60)90161-3. [DOI] [PubMed] [Google Scholar]
- WARREN L. The thiobarbituric acid assay of sialic acids. J Biol Chem. 1959 Aug;234(8):1971–1975. [PubMed] [Google Scholar]


