Abstract
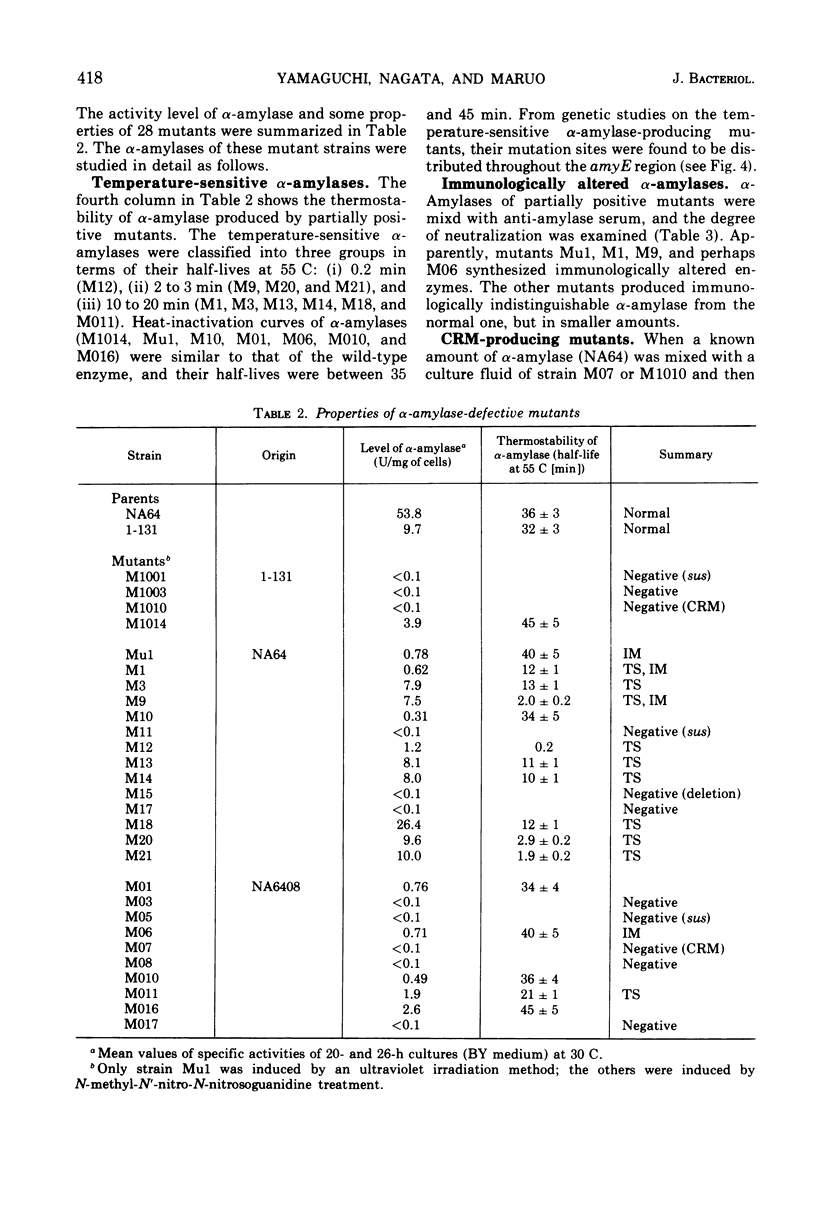
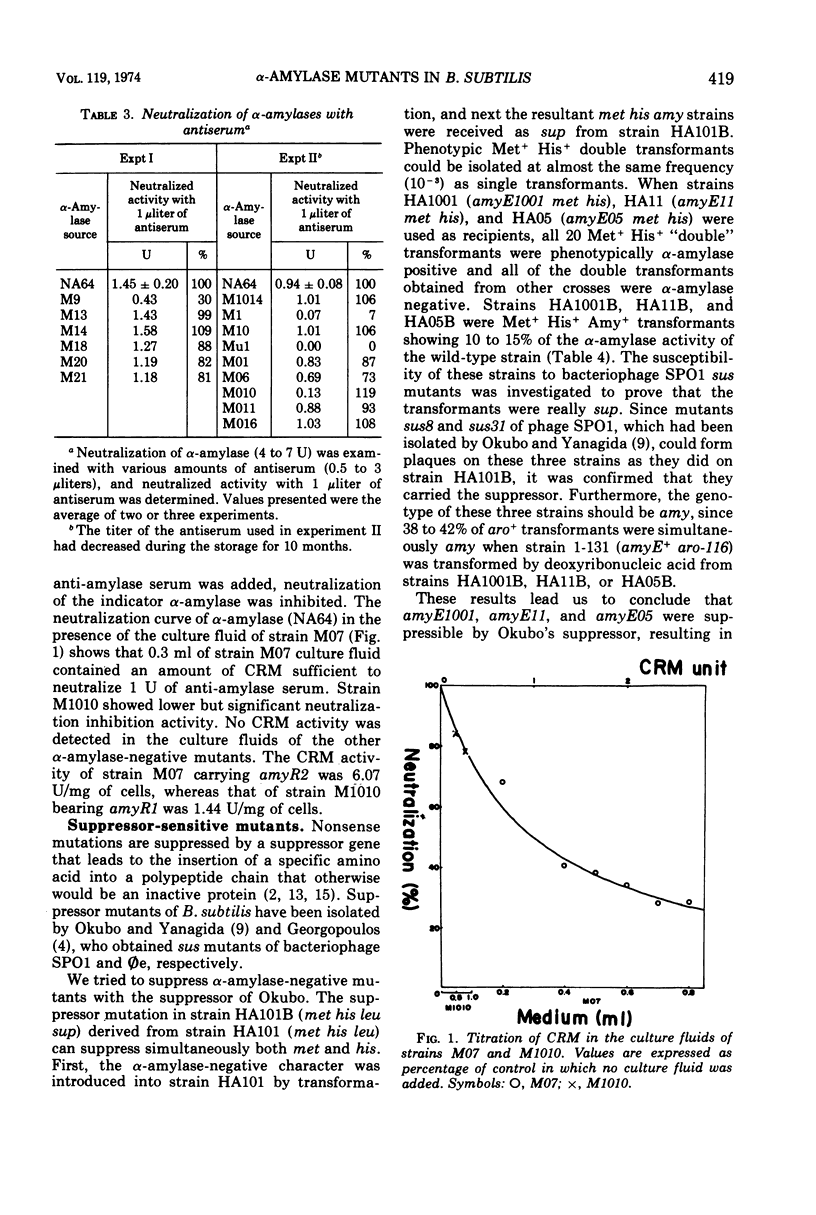
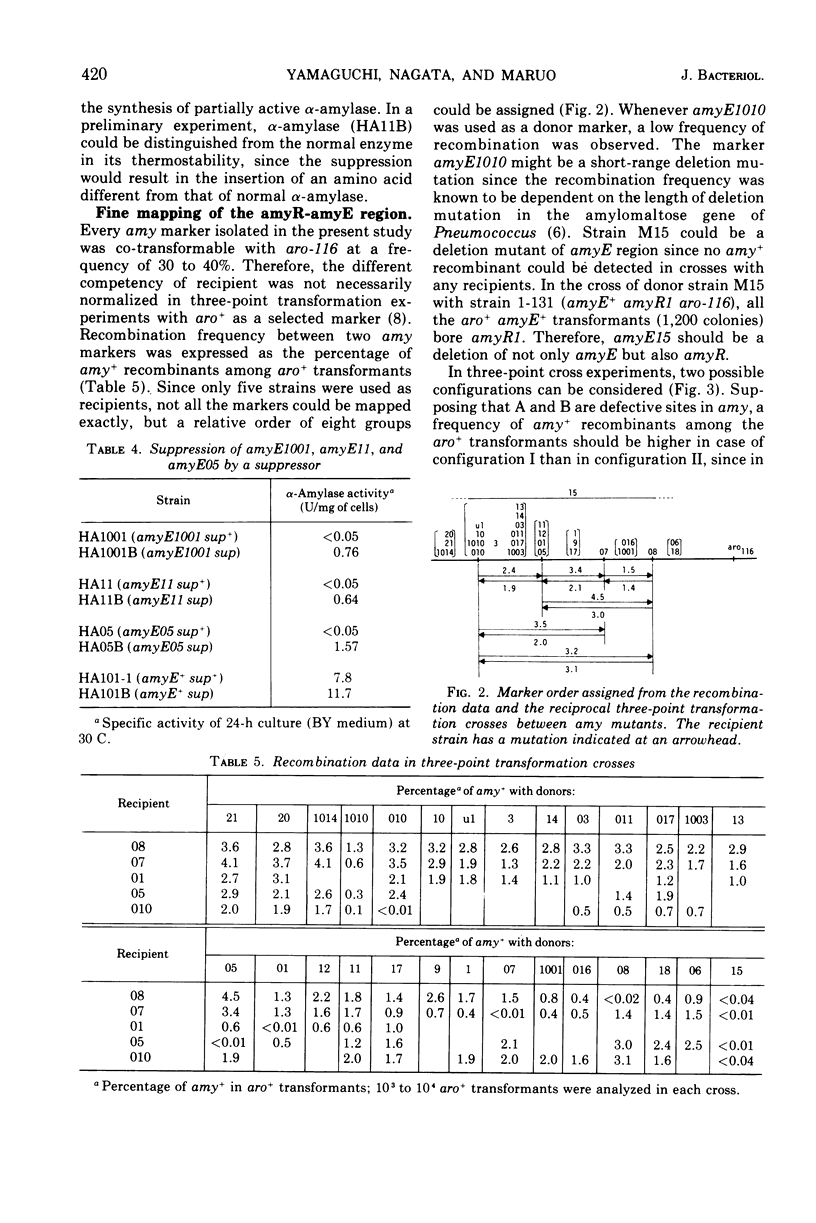
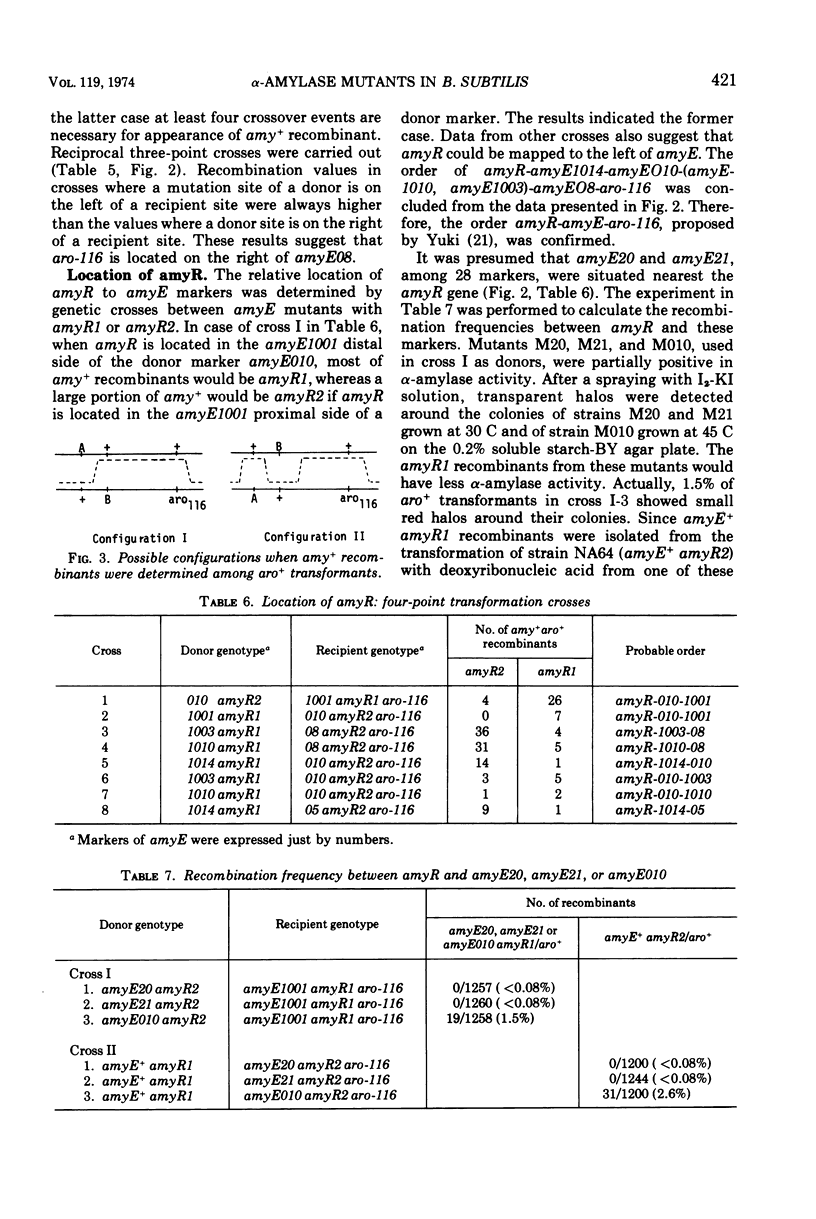
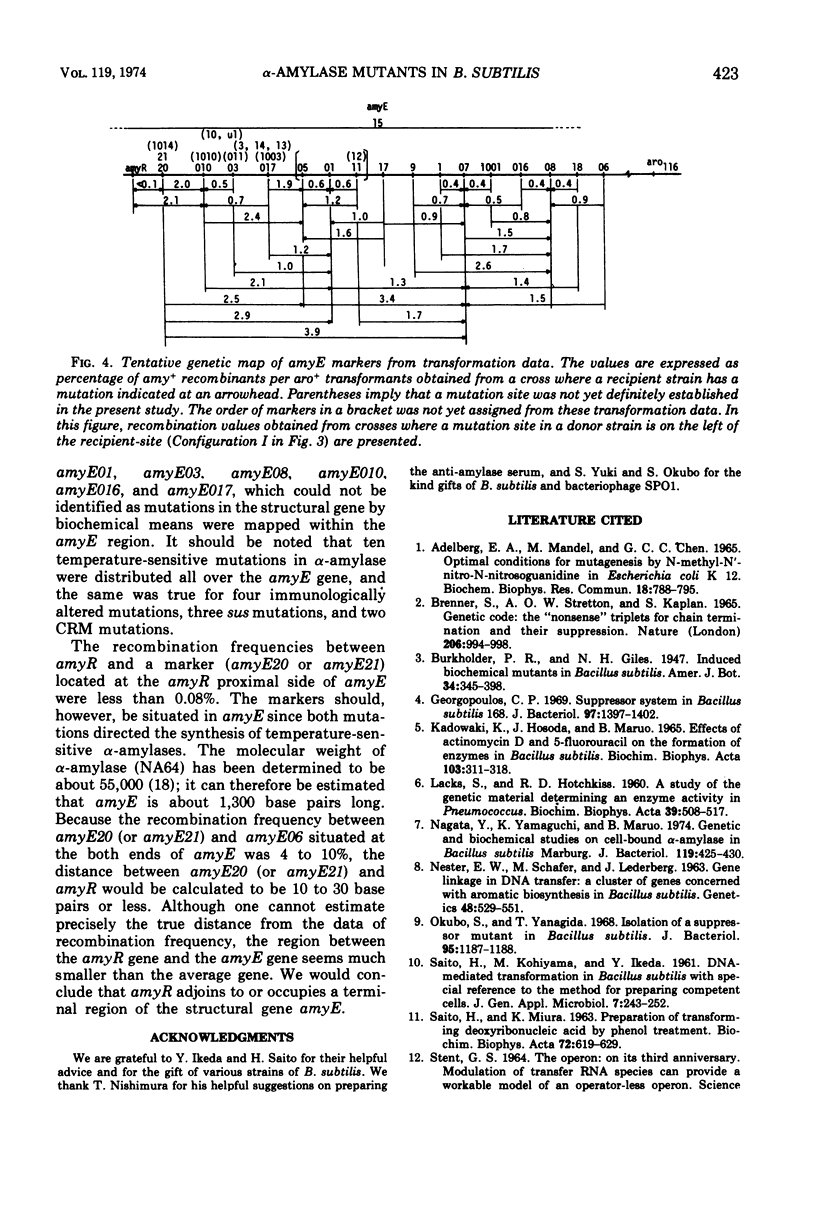
The rate of α-amylase (EC 3.2.1.1) synthesis in Bacillus subtilis is regulated by a gene, amyR, located near a structural gene, amyE, for the enzyme. To construct a fine map of the amyR-amyE region, we isolated 28 mutants defective in α-amylase activity. Eleven mutants out of 28 showed no α-amylase activity, whereas the other 17 showed less α-amylase activity than the parent. Out of 17 partially positive α-amylase mutants, 10 produced temperature-sensitive enzymes, and 4 produced immunologically altered enzymes, two of which are concurrently temperature-sensitive, and 5 produced smaller amounts of α-amylases which are indistinguishable from normal enzyme in their temperature sensitivity and immunological properties. Two out of 11 α-amylase-negative mutants produced material that cross-reacted with anti-amylase serum, and 3 mutants carried suppressible mutations by the suppressor described by Okubo. Mapping data indicate that all 28 mutation sites are located in the amyE region, and none of the groups of the mutants mentioned above contains lesions that are clustered in a single region of amyE. The amyR gene seems most likely to adjoin the terminal region of amyE.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brenner S., Stretton A. O., Kaplan S. Genetic code: the 'nonsense' triplets for chain termination and their suppression. Nature. 1965 Jun 5;206(988):994–998. doi: 10.1038/206994a0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Suppressor system in Bacillus subtilis 168. J Bacteriol. 1969 Mar;97(3):1397–1402. doi: 10.1128/jb.97.3.1397-1402.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadowaki K., Hosoda J., Maruo B. Effects of actinomycin D and 5-fluorouracil on the formation of enzymes in Bacillus subtilis. Biochim Biophys Acta. 1965 Jun 8;103(2):311–318. doi: 10.1016/0005-2787(65)90170-x. [DOI] [PubMed] [Google Scholar]
- LACKS S., HOTCHKISS R. D. A study of the genetic material determining an enzyme in Pneumococcus. Biochim Biophys Acta. 1960 Apr 22;39:508–518. doi: 10.1016/0006-3002(60)90205-5. [DOI] [PubMed] [Google Scholar]
- Nagata Y., Yamaguchi K., Maruo B. Genetic and biochemical studies on cell-bound alpha-amylase in Bacillus subtilis Marburg. J Bacteriol. 1974 Aug;119(2):425–430. doi: 10.1128/jb.119.2.425-430.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nester E W, Schafer M, Lederberg J. Gene Linkage in DNA Transfer: A Cluster of Genes Concerned with Aromatic Biosynthesis in Bacillus Subtilis. Genetics. 1963 Apr;48(4):529–551. doi: 10.1093/genetics/48.4.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S., Yanagida T. Isolation of a suppressor mutant in Bacillus subtilis. J Bacteriol. 1968 Mar;95(3):1187–1188. doi: 10.1128/jb.95.3.1187-1188.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- STRETTON A. O., BRENNER S. MOLECULAR CONSEQUENCES OF THE AMBER MUTATION AND ITS SUPPRESSION. J Mol Biol. 1965 Jun;12:456–465. doi: 10.1016/s0022-2836(65)80268-6. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEIGERT M. G., GAREN A. AMINO ACID SUBSTITUTIONS RESULTING FROM SUPPRESSION OF NONSENSE MUTATIONS. I. SERINE INSERTION BY THE SU-1 SUPPRESSOR GENE. J Mol Biol. 1965 Jun;12:448–455. doi: 10.1016/s0022-2836(65)80267-4. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Nagata Y., Maruo B. Genetic control of the rate of alpha-amylase synthesis in Bacillus subtilis. J Bacteriol. 1974 Aug;119(2):410–415. doi: 10.1128/jb.119.2.410-415.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K., Yamaguchi K., Maruo B. Purification and properties of a cross-reacting material related to -amylase and biochemical comparison with the parent -amylase. Biochim Biophys Acta. 1973 Jan 25;295(1):323–340. doi: 10.1016/0005-2795(73)90100-1. [DOI] [PubMed] [Google Scholar]
- Yoneda Y., Yamane K., Maruo B. Membrane mutation related to the production of extracellular -amylase and protease in bacillus subtilis. Biochem Biophys Res Commun. 1973 Feb 5;50(3):765–770. doi: 10.1016/0006-291x(73)91310-7. [DOI] [PubMed] [Google Scholar]
- Yoshikawa H. DNA synthesis during germination of Bacillus subtilis spores. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1476–1483. doi: 10.1073/pnas.53.6.1476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuki S. On the gene controlling the rate of amylase production in Bacillus subtilis. Biochem Biophys Res Commun. 1968 Apr 19;31(2):182–187. doi: 10.1016/0006-291x(68)90727-4. [DOI] [PubMed] [Google Scholar]


