Abstract
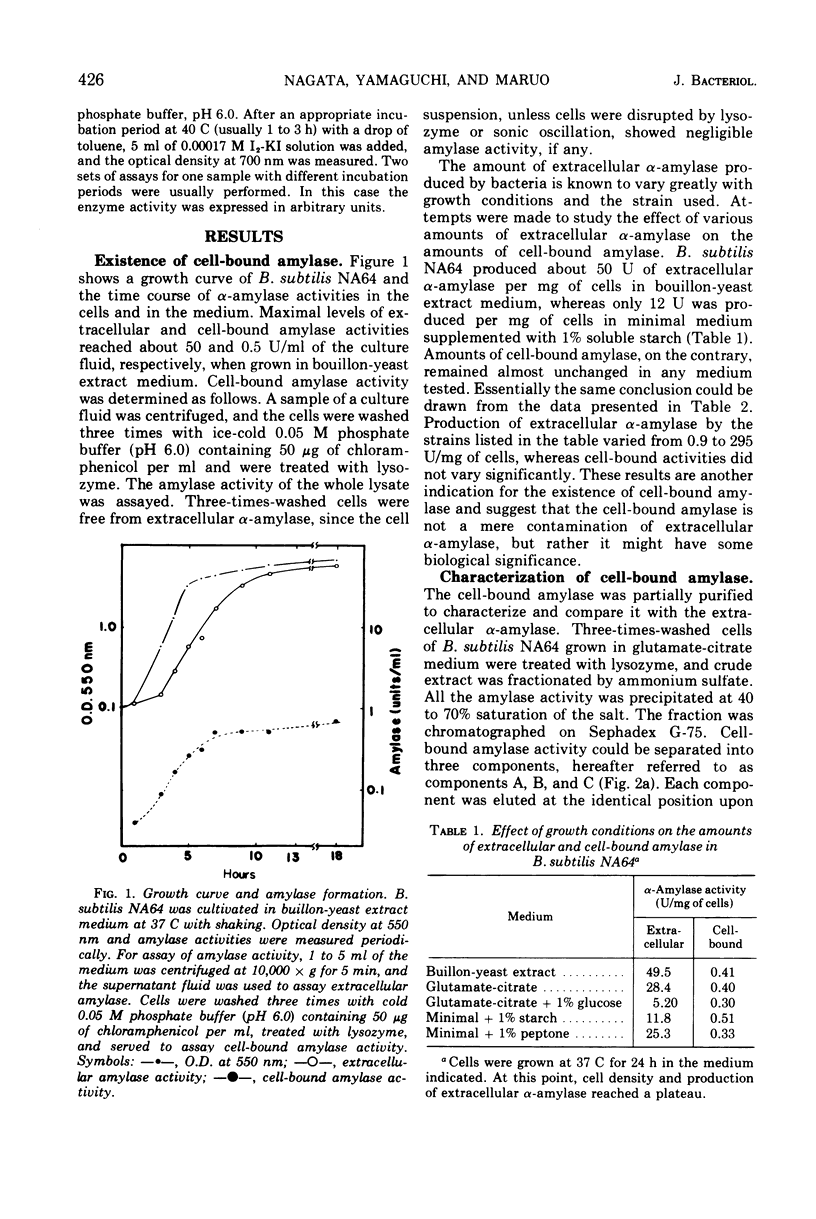
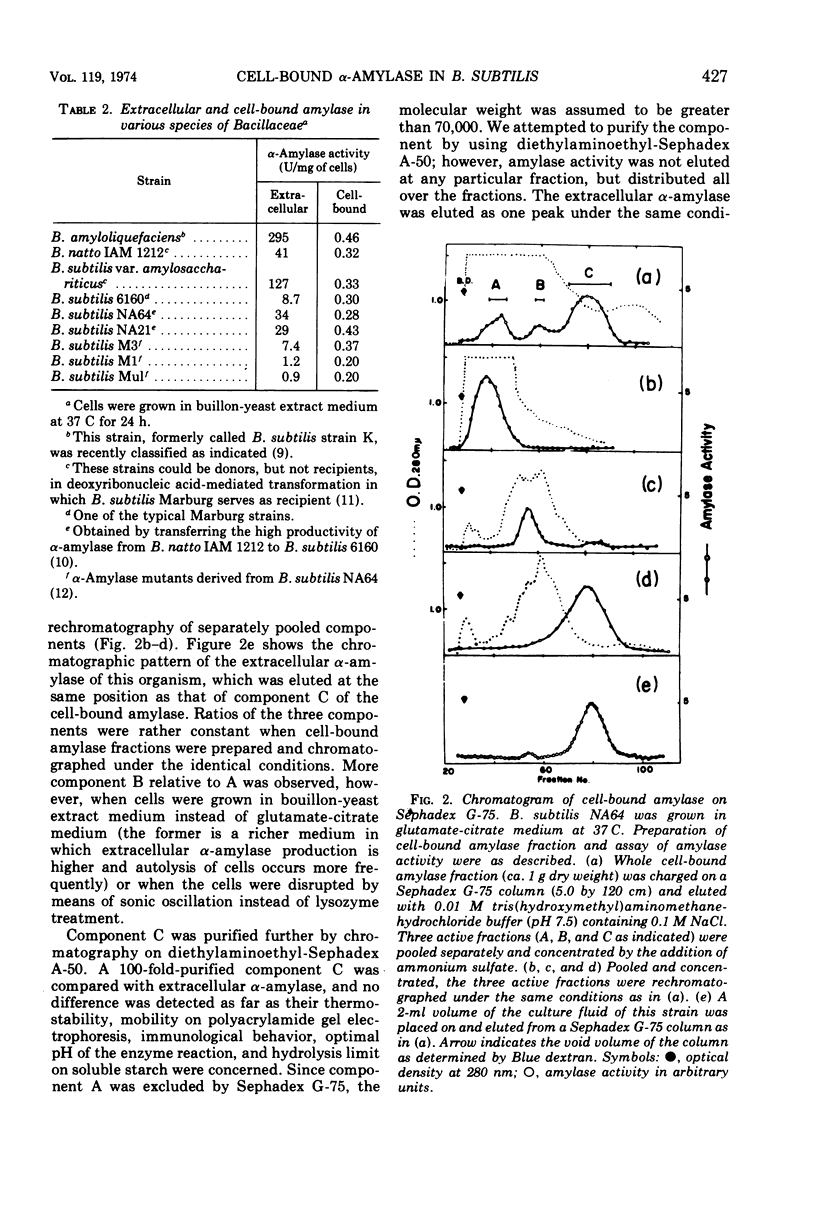
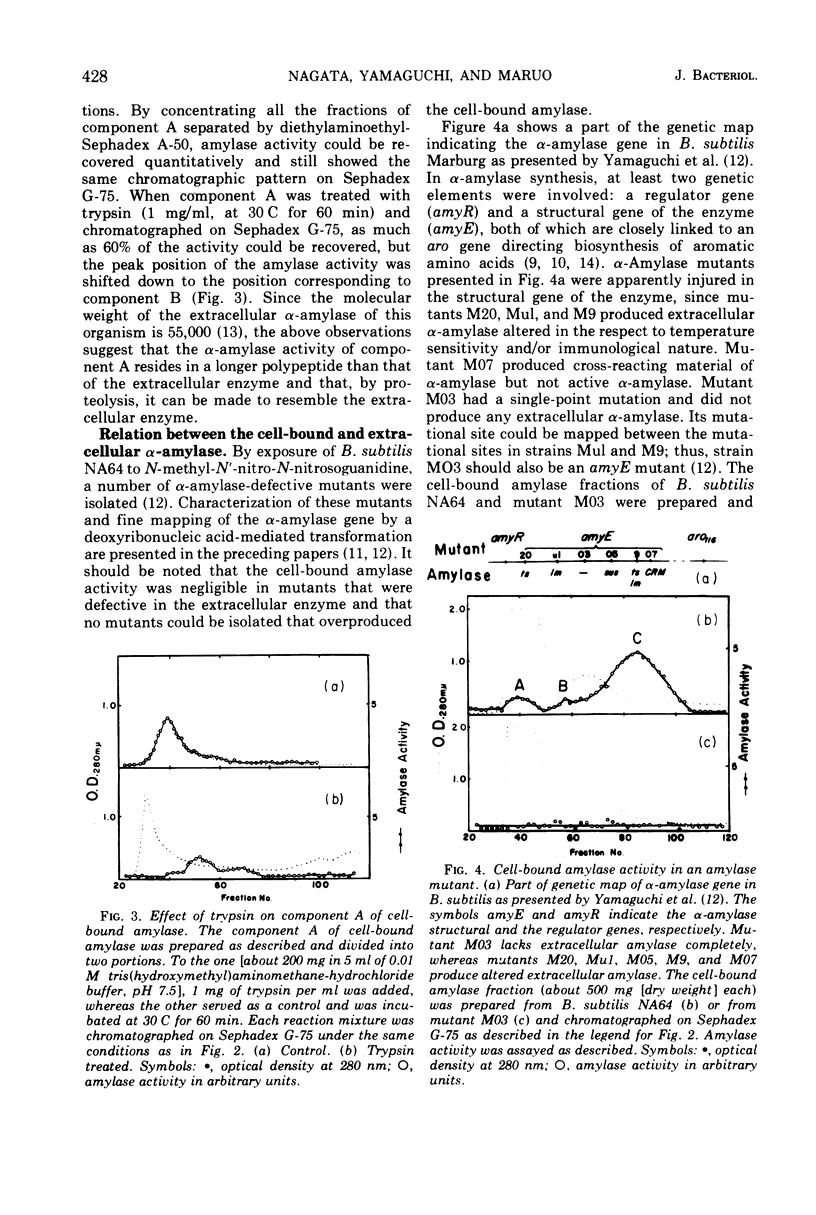
A small but significant amount of α-amylase activity was detected in the cells of Bacillus subtilis Marburg. The cell-associated activity was almost constant regardless of the level of extracellular α-amylase activity. The cell-bound amylase activity could be separated into three components, upon Sephadex G-75 chromatography, referred to as components A, B, and C. Component C showed the same properties as the extracellular α-amylases so far examined. Component A had a molecular weight greater than 70,000, as judged from the elution position on Sephadex G-75, and became smaller upon treatment with trypsin but was still larger than that of component C. An α-amylase mutant that lacked extracellular α-amylase completely because of a mutation within the structural gene of the enzyme was found to lose all three cell-bound amylase components simultaneously. These data suggest strongly that the cell-bound amylase components are precursors of the extracellular α-amylase and that the α-amylase of this organism is produced under the direction of the same gene whether the enzyme is within or outside the cell.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COLEMAN G., ELLIOTT W. H. Studies on alpha-amylase formation by Bacillus subtilis. Biochem J. 1962 May;83:256–263. doi: 10.1042/bj0830256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles N. W., Gross R. Exopenicillinase synthesis in Staphylococcus aureus. J Bacteriol. 1969 May;98(2):659–661. doi: 10.1128/jb.98.2.659-661.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampen J. O. Cell-bound penicillinase of Bacillus licheniformis; properties and purification. J Gen Microbiol. 1967 Aug;48(2):249–259. doi: 10.1099/00221287-48-2-249. [DOI] [PubMed] [Google Scholar]
- OISHI M., TAKAHASHI H., MARUO B. Intracellular alpha-amylase in Bacillus subtilis. J Bacteriol. 1963 Jan;85:246–247. doi: 10.1128/jb.85.1.246-247.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Lampen J. O. A mechanism for penicillinasesecretion in Bacillus licheniformis. Proc Natl Acad Sci U S A. 1970 Apr;65(4):962–969. doi: 10.1073/pnas.65.4.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sargent M. G., Lampen J. O. Organization of the membrane-bound penicillinases of Bacillus licheniformis. Arch Biochem Biophys. 1970 Jan;136(1):167–177. doi: 10.1016/0003-9861(70)90338-3. [DOI] [PubMed] [Google Scholar]
- THAYER P. S. The amylases of pseudomonas saccharophila. J Bacteriol. 1953 Dec;66(6):656–663. doi: 10.1128/jb.66.6.656-663.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welker N. E., Campbell L. L. Unrelatedness of Bacillus amyloliquefaciens and Bacillus subtilis. J Bacteriol. 1967 Oct;94(4):1124–1130. doi: 10.1128/jb.94.4.1124-1130.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Nagata Y., Maruo B. Genetic control of the rate of alpha-amylase synthesis in Bacillus subtilis. J Bacteriol. 1974 Aug;119(2):410–415. doi: 10.1128/jb.119.2.410-415.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamaguchi K., Nagata Y., Maruo B. Isolation of mutants defective in alpha-amylase from Bacillus subtilis: genetic analyses. J Bacteriol. 1974 Aug;119(2):416–424. doi: 10.1128/jb.119.2.416-424.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamane K., Yamaguchi K., Maruo B. Purification and properties of a cross-reacting material related to -amylase and biochemical comparison with the parent -amylase. Biochim Biophys Acta. 1973 Jan 25;295(1):323–340. doi: 10.1016/0005-2795(73)90100-1. [DOI] [PubMed] [Google Scholar]
- Yoneda Y., Yamane K., Maruo B. Membrane mutation related to the production of extracellular -amylase and protease in bacillus subtilis. Biochem Biophys Res Commun. 1973 Feb 5;50(3):765–770. doi: 10.1016/0006-291x(73)91310-7. [DOI] [PubMed] [Google Scholar]


