Abstract
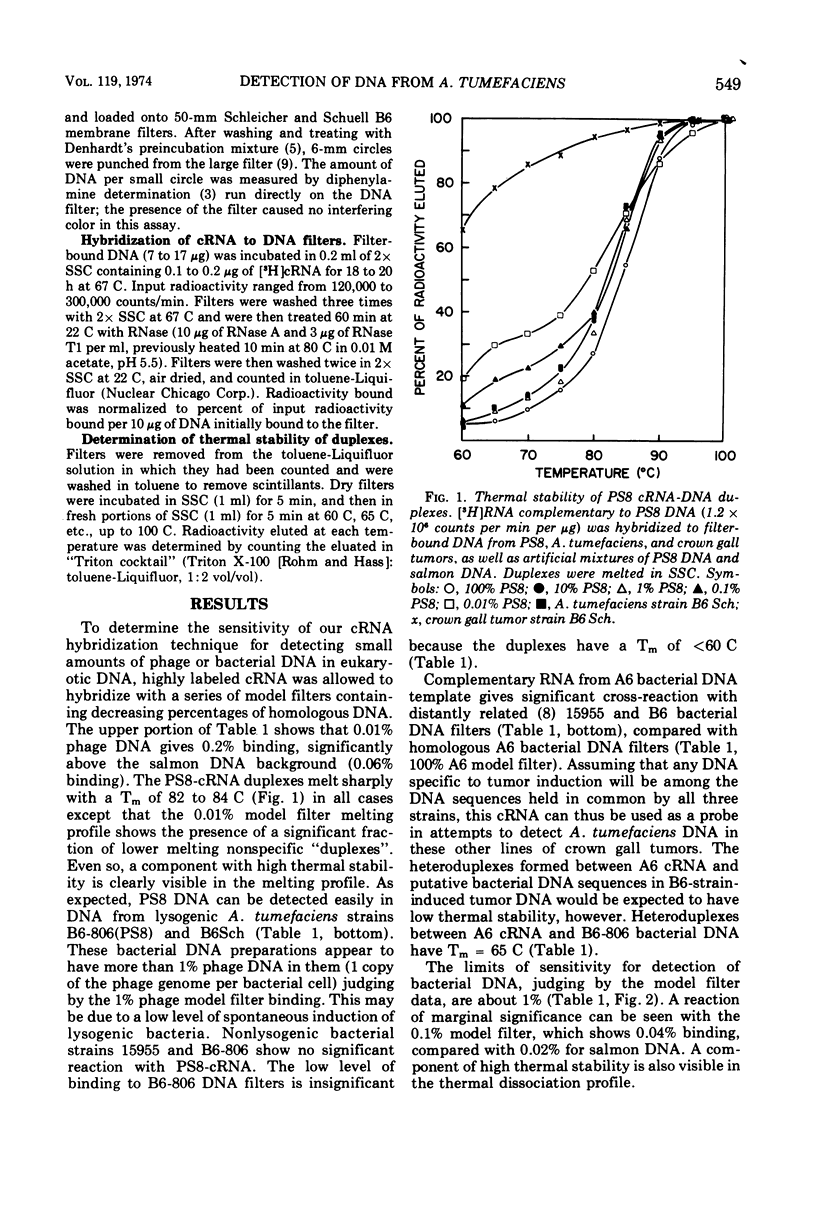
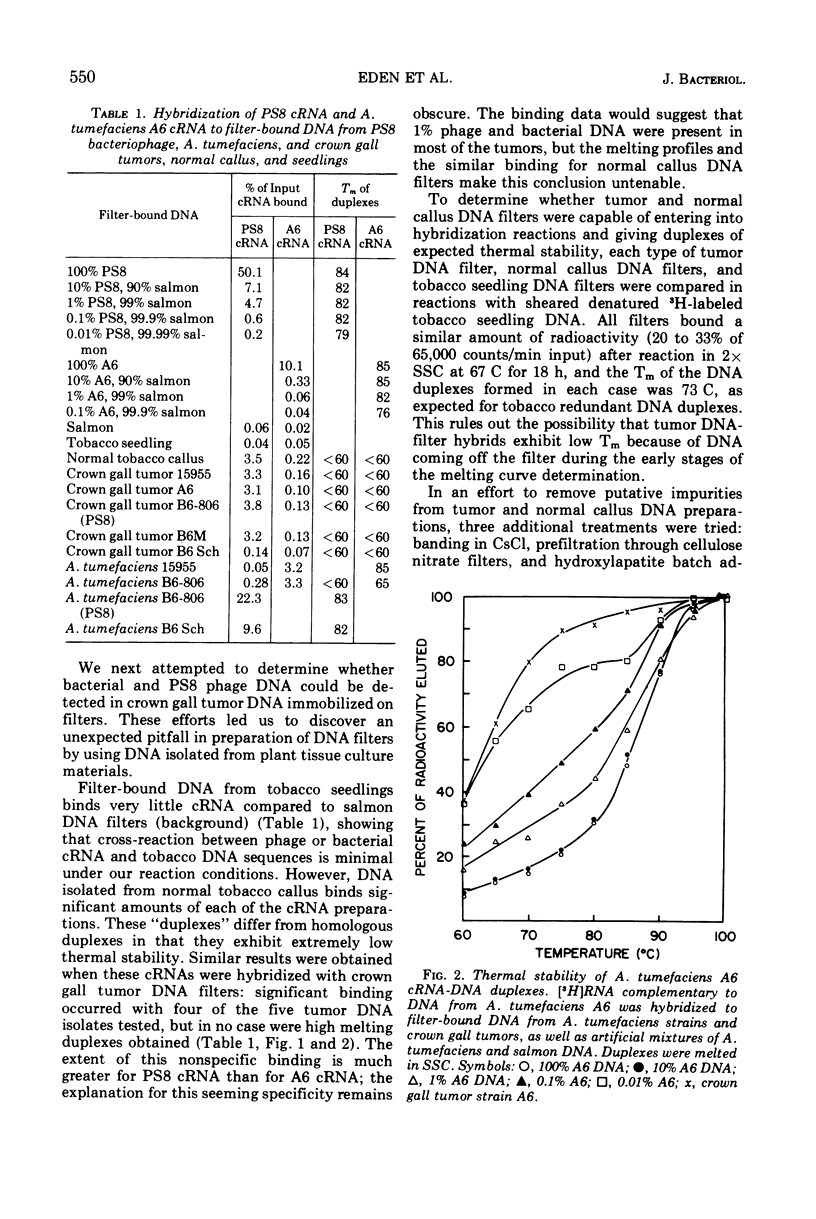
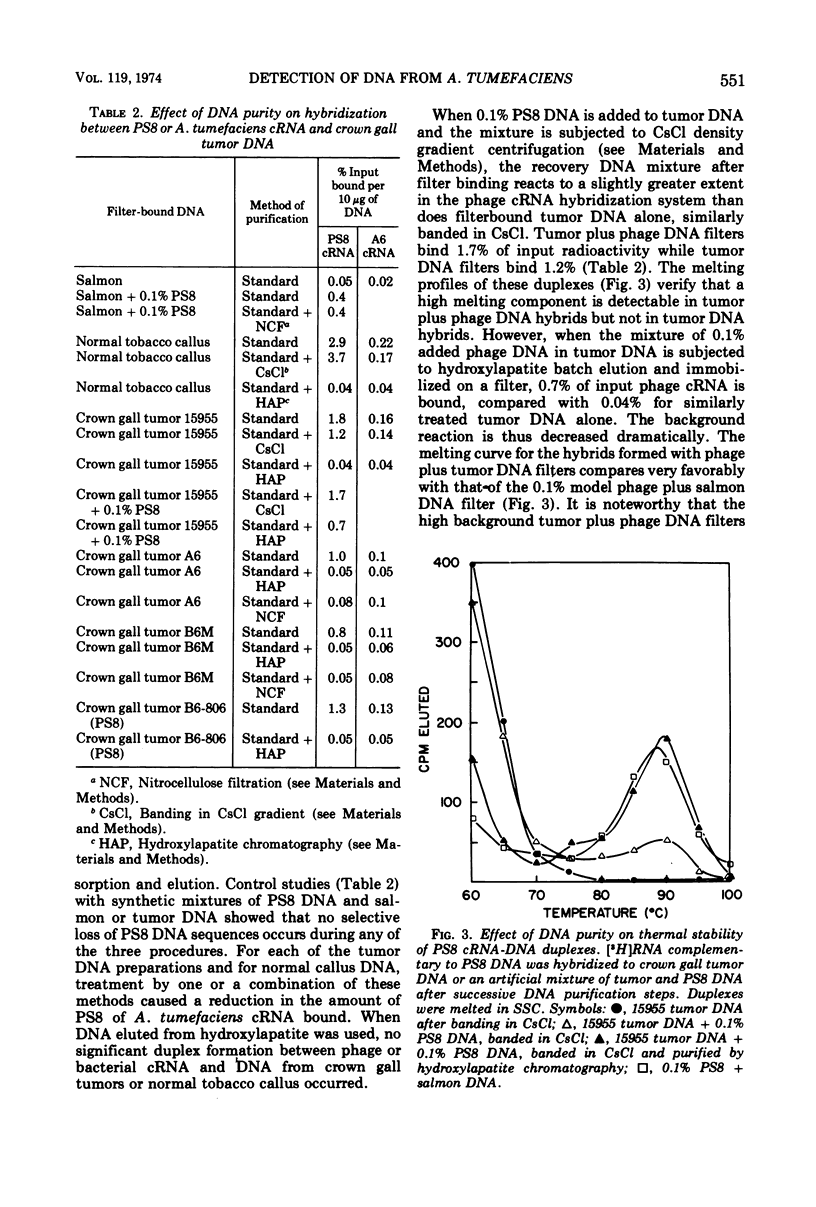
Labeled ribonucleic acid (RNA) complementary to Agrobacterium tumefaciens DNA and PS8 bacteriophage DNA (cRNA) were used in a systematic study of the sensitivity of cRNA/deoxyribonucleic acid (DNA)-filter hybridization for detection of small amounts of phage or bacterial DNA immobilized on filters. A. tumefaciens cRNA of specific activity 106 to 2 × 106 counts per min per μg reacted to a significant extent when the DNA-filter contained 1% A. tumefaciens DNA in a salmon DNA background, but 0.1% A. tumefaciens DNA was not detectable. PS8 phage cRNA of the same specific activity reacted to a significant extent when the DNA-filter contained as little as 0.01% PS8 DNA in a salmon DNA background. Both kinds of cRNA were found to bind to tobacco crown gall tumor DNA-filters. Similar reaction was found with control normal callus DNA-filters but not with tobacco seedling DNA-filters. The “hybrids” formed by cRNA with normal callus and tumor DNA-filters had low thermal stability. Attempts to purify the tumor and normal callus DNA prior to immobilization on the filter resulted in elimination of this spurious binding. No evidence was found for bacterial or phage DNA in crown gall tumor DNA.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnstiel M. L., Sells B. H., Purdom I. F. Kinetic complexity of RNA molecules. J Mol Biol. 1972 Jan 14;63(1):21–39. doi: 10.1016/0022-2836(72)90519-0. [DOI] [PubMed] [Google Scholar]
- Britten R. J., Kohne D. E. Repeated sequences in DNA. Hundreds of thousands of copies of DNA sequences have been incorporated into the genomes of higher organisms. Science. 1968 Aug 9;161(3841):529–540. doi: 10.1126/science.161.3841.529. [DOI] [PubMed] [Google Scholar]
- CHAMBERLIN M., BERG P. Deoxyribo ucleic acid-directed synthesis of ribonucleic acid by an enzyme from Escherichia coli. Proc Natl Acad Sci U S A. 1962 Jan 15;48:81–94. doi: 10.1073/pnas.48.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denhardt D. T. A membrane-filter technique for the detection of complementary DNA. Biochem Biophys Res Commun. 1966 Jun 13;23(5):641–646. doi: 10.1016/0006-291x(66)90447-5. [DOI] [PubMed] [Google Scholar]
- Hill E. B., Wayne L. G., Gross W. M. Purification of mycobacterial deoxyribonucleic acid. J Bacteriol. 1972 Dec;112(3):1033–1039. doi: 10.1128/jb.112.3.1033-1039.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson R., Guderian R. H., Eden F., Chilton M. D., Gordon M. P., Nester E. W. Detection and quantitation of octopine in normal plant tissue and in crown gall tumors. Proc Natl Acad Sci U S A. 1974 Feb;71(2):536–539. doi: 10.1073/pnas.71.2.536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy B. J., McConaughy B. L. Related base sequences in the DNA of simple and complex organisms. I. DNA-DNA duplex formation and the incidence of partially related base sequences in DNA. Biochem Genet. 1968 Jun;2(1):37–53. doi: 10.1007/BF01458450. [DOI] [PubMed] [Google Scholar]
- Quétier F., Huguet T., Guillé E. Induction of Crown-gall: partial homology between tumor-cell DNA, bacterial DNA and the G+C--rich DNA of stressed normal cells. Biochem Biophys Res Commun. 1969 Jan 6;34(1):128–133. doi: 10.1016/0006-291x(69)90538-5. [DOI] [PubMed] [Google Scholar]
- Schilperoort R. A., Van Sittert N. J., Schell J. The presence of both phage PS8 and Agrobacterium tumefaciens A 6 DNA base sequences in A 6 -induced sterile crown-gall tissue cultured in vitro. Eur J Biochem. 1973 Feb 15;33(1):1–7. doi: 10.1111/j.1432-1033.1973.tb02647.x. [DOI] [PubMed] [Google Scholar]
- Schilperoort R. A., Veldstra H., Warnaar S. O., Mulder G., Cohen J. A. Formation of complexes between DNA isolated from tobacco crown gall tumours and RNA complementary to Agrobacterium tumefaciens DNA. Biochim Biophys Acta. 1967 Sep 26;145(2):523–525. doi: 10.1016/0005-2787(67)90075-5. [DOI] [PubMed] [Google Scholar]
- Siegel A., Lightfoot D., Ward O. G., Keener S. DNA Complementary to Ribosomal RNA: Relation between Genomic Proportion and Ploidy. Science. 1973 Feb 16;179(4074):682–683. doi: 10.1126/science.179.4074.682. [DOI] [PubMed] [Google Scholar]
- Srivastava B. I. DNA-DNA hybridization studies between bacterial DNA, crown gall tumor cell DNA and the normal cell DNA. Life Sci II. 1970 Aug 8;9(15):889–892. doi: 10.1016/0024-3205(70)90058-5. [DOI] [PubMed] [Google Scholar]
- Whiteley A. H., McCarthy B. J., Whiteley H. R. Changing populations of messenger RNA during sea urchin development. Proc Natl Acad Sci U S A. 1966 Mar;55(3):519–525. doi: 10.1073/pnas.55.3.519. [DOI] [PMC free article] [PubMed] [Google Scholar]


