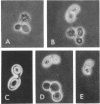
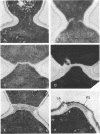
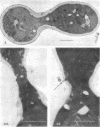
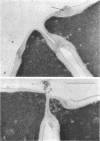
Abstract
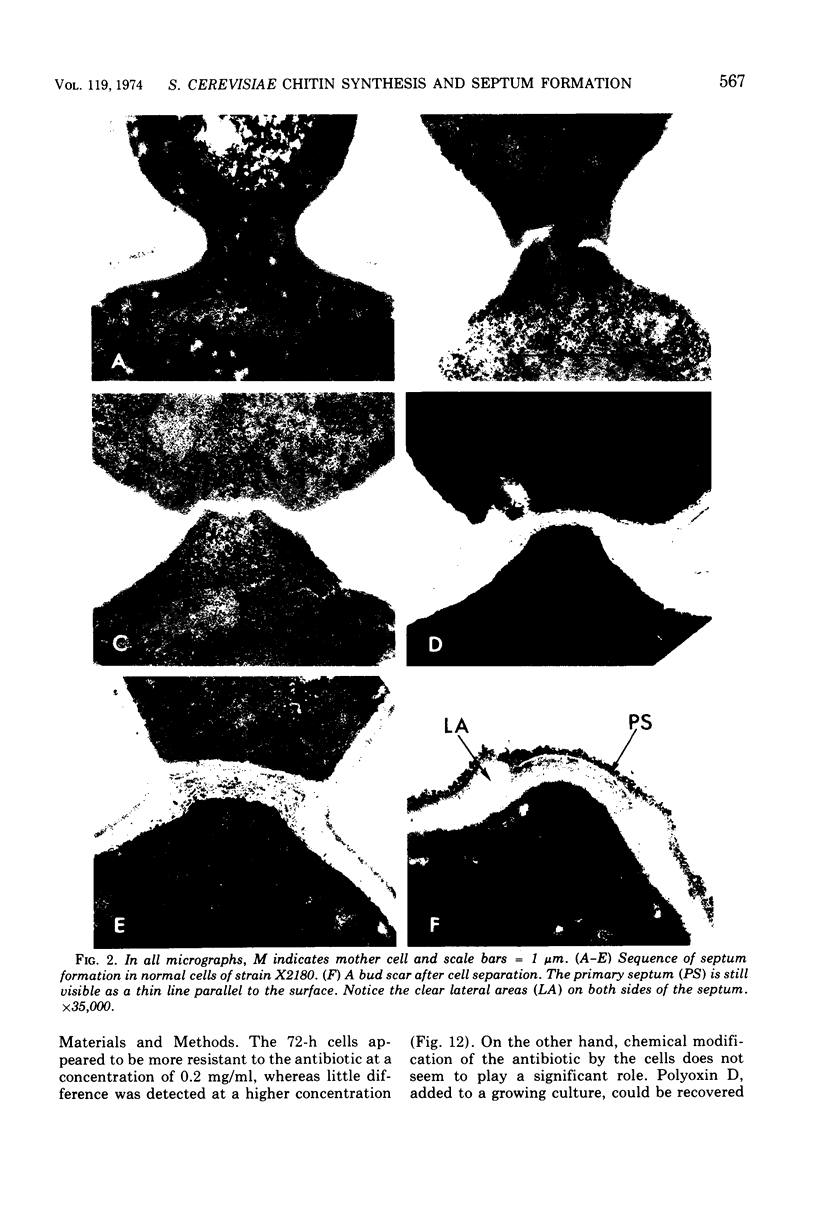
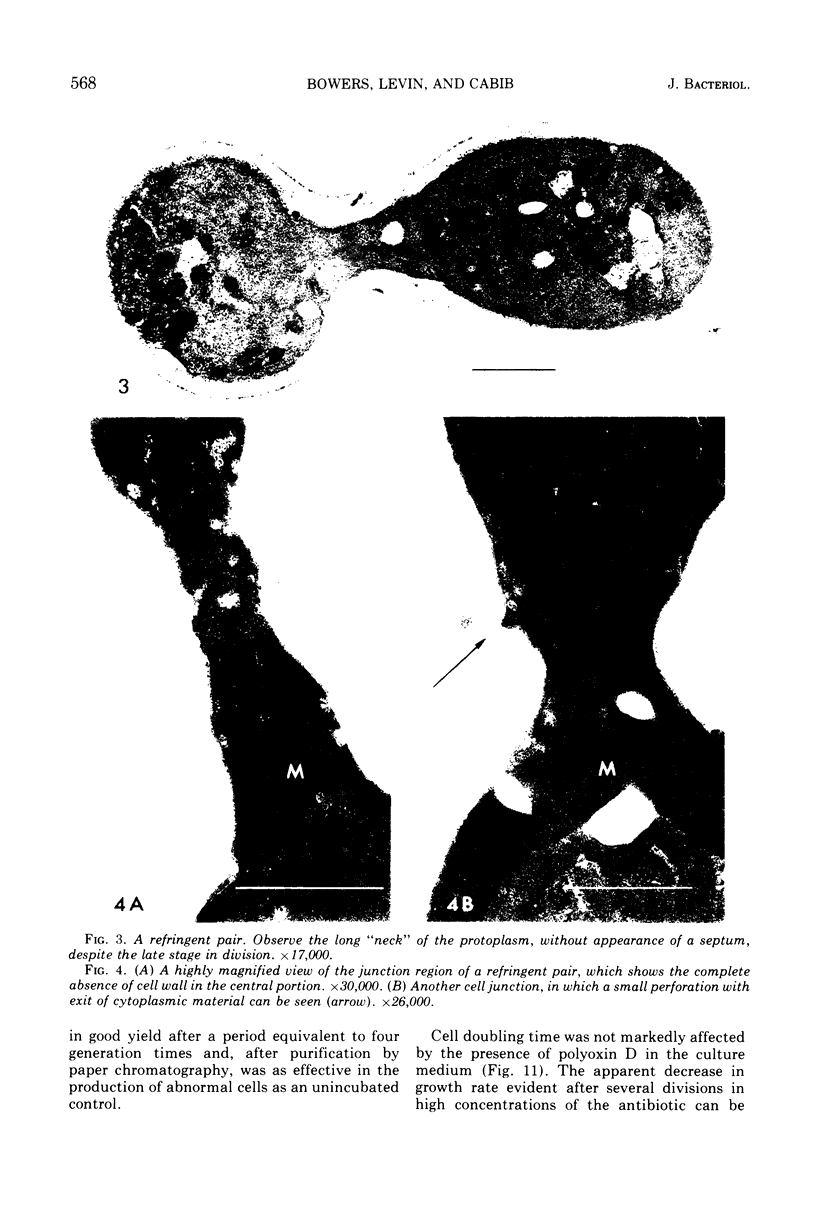
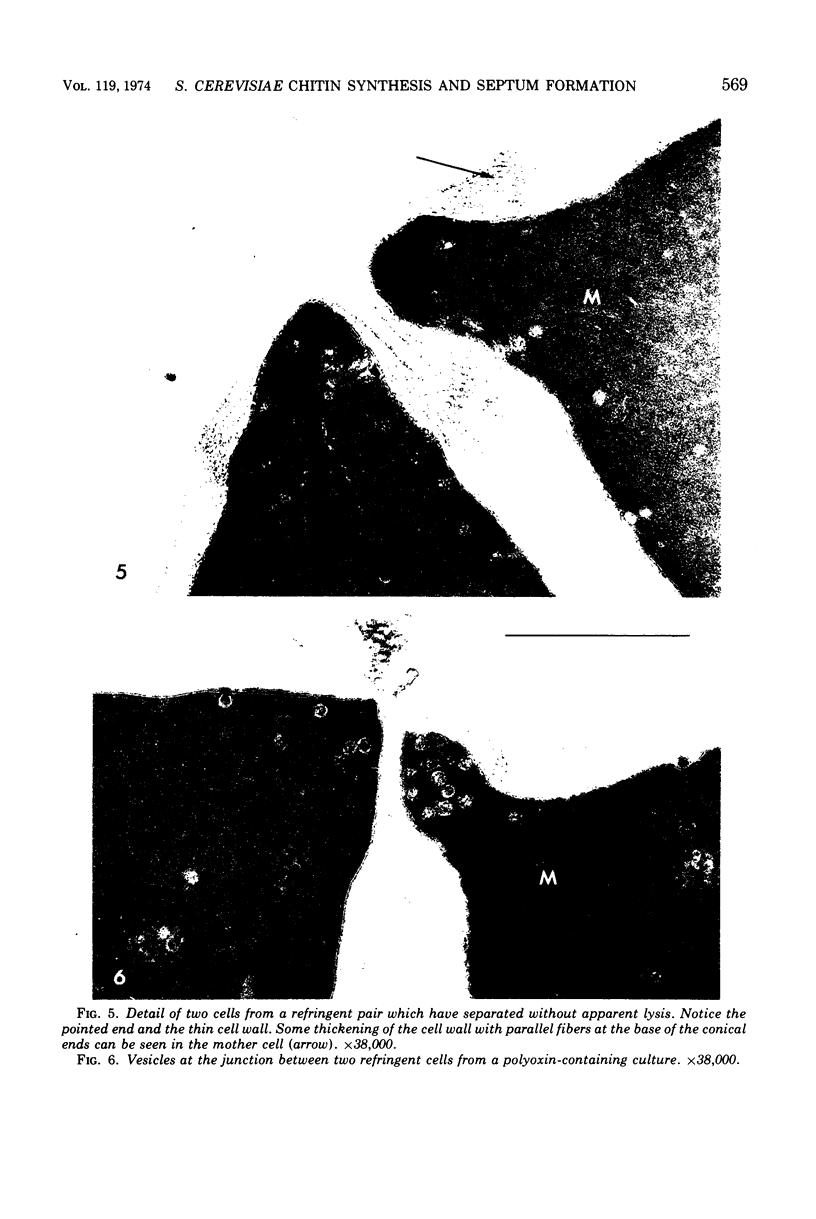
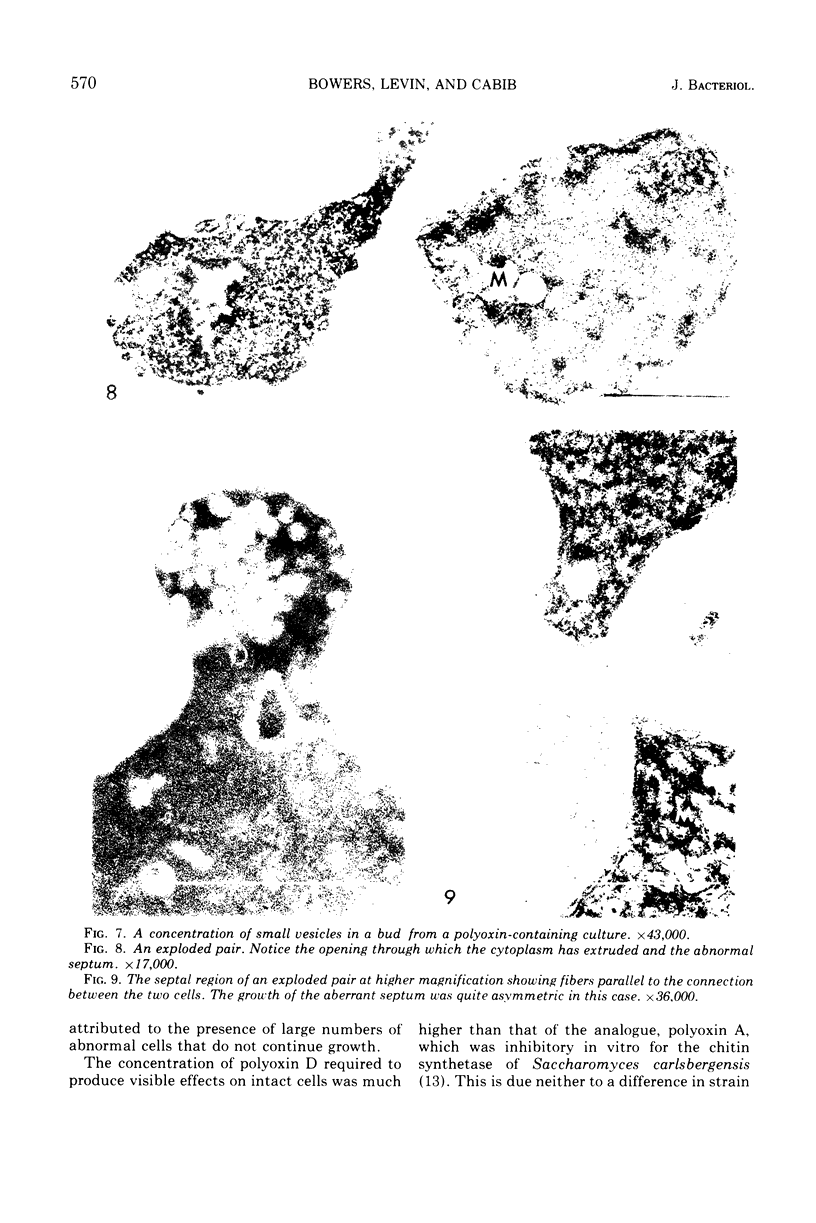
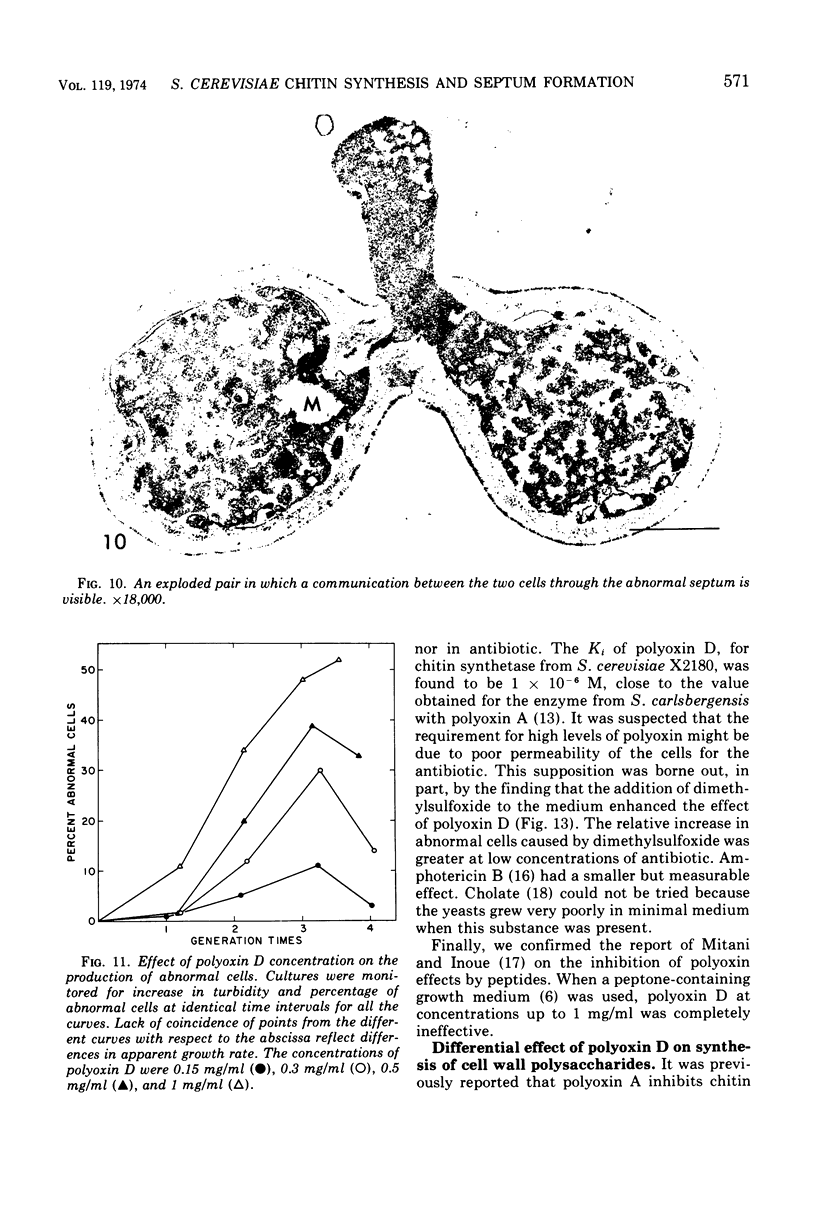
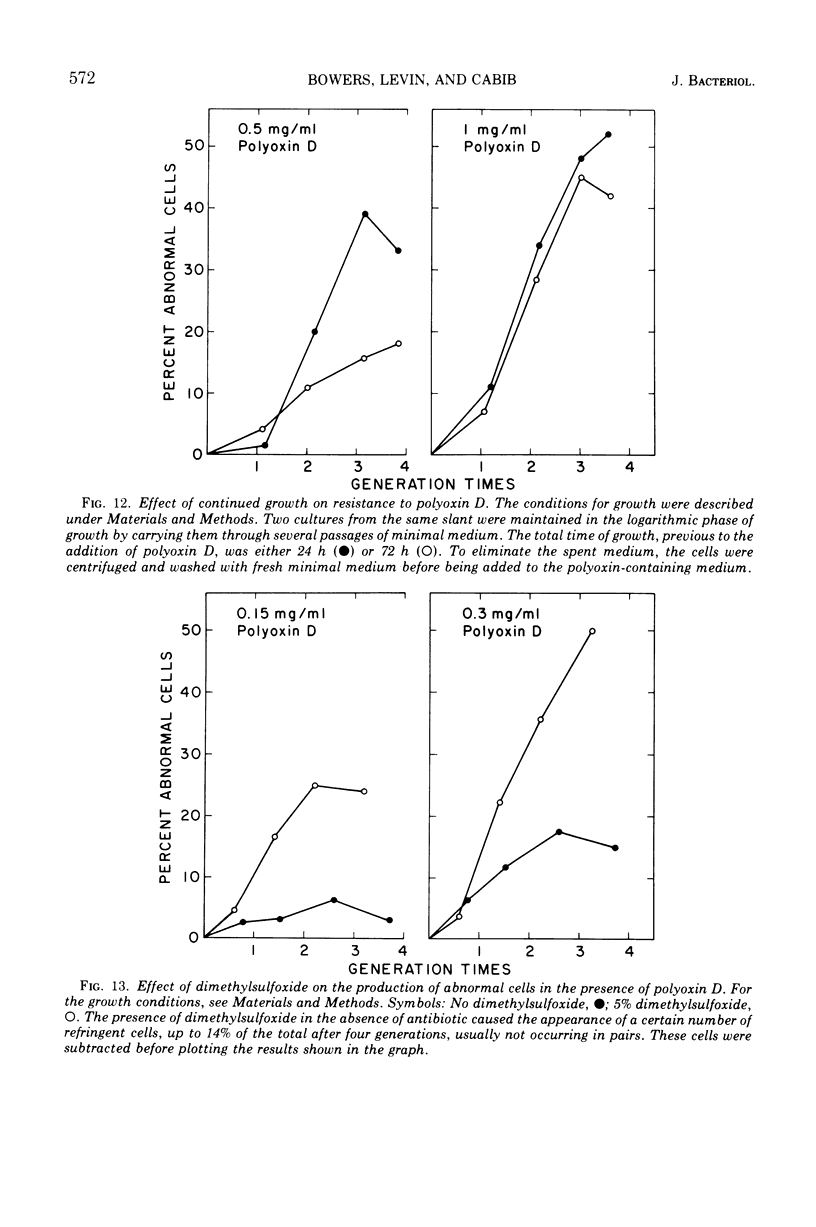
The normal sequence of cell separation in Saccharomyces cerevisiae begins with the formation of a primary septum, presumably consisting of chitin, on which secondary septa are later deposited. In the presence of the antibiotic polyoxin D, a potent inhibitor of chitin synthetase, pairs of abnormal cells of two different types were observed by phase-contrast microscopy: the “exploded pair,” consisting of two lysed cells from which the cytoplasm had been extruded at the cell junction, and the “refringent pair,” consisting of two highly refractile cells joined by a thin bridge. Thus, in both cases the septal region appears to be affected. Observations with the electron microscope showed that the primary chitin septum was not formed in either of these cell types, and as a consequence secondary septa of varying thicknesses were laid down in an abnormal pattern. With [3H]glucose as carbon source the incorporation of tritium into the chitin of abnormal cells was inhibited about 90%, whereas the labeling of mannan was normal and that of glucan somewhat reduced. The effective concentrations of polyoxin D (0.1 to 1 mg/ml) were much greater than those required to inhibit chitin synthesis in vitro. Dimethylsulfoxide and amphotericin B, both known to increase cell permeability, enhanced the action of the antibiotic.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon J. S., Farmer V. C., Jones D., Taylor I. F. The glucan components of the cell wall of baker's yeast (Saccharomyces cerevisiae) considered in relation to its ultrastructure. Biochem J. 1969 Sep;114(3):557–567. doi: 10.1042/bj1140557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin J. M. A study of the budding of Saccharomyces uvarum Beijerinck with the scanning electron microscope. Antonie Van Leeuwenhoek. 1972;38(3):341–349. doi: 10.1007/BF02328103. [DOI] [PubMed] [Google Scholar]
- Cabib E., Bowers B. Chitin and yeast budding. Localization of chitin in yeast bud scars. J Biol Chem. 1971 Jan 10;246(1):152–159. [PubMed] [Google Scholar]
- Cabib E., Farkas V. The control of morphogenesis: an enzymatic mechanism for the initiation of septum formation in yeast. Proc Natl Acad Sci U S A. 1971 Sep;68(9):2052–2056. doi: 10.1073/pnas.68.9.2052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortat M., Matile P., Wiemken A. Isolation of glucanase-containing vesicles from budding yeast. Arch Mikrobiol. 1972;82(3):189–205. doi: 10.1007/BF00412191. [DOI] [PubMed] [Google Scholar]
- Endo A., Kakiki K., Misato T. Mechanism of action of the antifugal agent polyoxin D. J Bacteriol. 1970 Oct;104(1):189–196. doi: 10.1128/jb.104.1.189-196.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Periodic density fluctuation during the yeast cell cycle and the selection of synchronous cultures. J Bacteriol. 1970 Dec;104(3):1280–1285. doi: 10.1128/jb.104.3.1280-1285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller F. A., Cabib E. Chitin and yeast budding. Properties of chitin synthetase from Saccharomyces carlsbergensis. J Biol Chem. 1971 Jan 10;246(1):160–166. [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant R., Smith D. G. Bud formation in Saccharomyces cerevisiae and a comparison with the mechanism of cell division in other yeasts. J Gen Microbiol. 1968 Sep;53(2):163–169. doi: 10.1099/00221287-53-2-163. [DOI] [PubMed] [Google Scholar]
- Medoff G., Kobayashi G. S., Kwan C. N., Schlessinger D., Venkov P. Potentiation of rifampicin and 5-fluorocytosine as antifungal antibiotics by amphotericin B (yeast-membrane permeability-ribosomal RNA-eukaryotic cell-synergism). Proc Natl Acad Sci U S A. 1972 Jan;69(1):196–199. doi: 10.1073/pnas.69.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitani M., Inoue Y. Antagonists of an antifungal substance, polyoxin. J Antibiot (Tokyo) 1968 Aug;21(8):492–496. doi: 10.7164/antibiotics.21.492. [DOI] [PubMed] [Google Scholar]
- Scholz K., Jaenicke L. Regelung der Synthese von Dihydrofolsäure-Reduktase bei synchronisierten Hefezellen. Eur J Biochem. 1968 May;4(4):448–457. doi: 10.1111/j.1432-1033.1968.tb00233.x. [DOI] [PubMed] [Google Scholar]
- Sentandreu R., Northcote D. H. The formation of buds in yeast. J Gen Microbiol. 1969 Mar;55(3):393–398. doi: 10.1099/00221287-55-3-393. [DOI] [PubMed] [Google Scholar]
- Shannon J. L., Rothman A. H. Transverse septum formation in budding cells of the yeastlike fungus Candida albicans. J Bacteriol. 1971 Jun;106(3):1026–1028. doi: 10.1128/jb.106.3.1026-1028.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]