Abstract
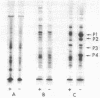
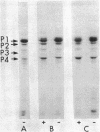
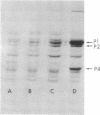
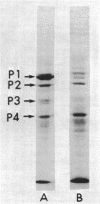
Induction of alkaline phosphatase in wild-type Escherichia coli K-12 leads to the appearance of three new proteins in addition to alkaline phosphatase in the periplasmic space of the bacteria. These proteins are detected in autoradiograms of sodium dodecyl sulfate-acrylamide gel electropherograms of extracts from cells labeled with [35S]methionine. Studies with constitutive mutants defective in the three genes phoS, phoT, and phoR that have been shown to regulate alkaline phosphatase synthesis indicate that the three periplasmic proteins are coregulated with alkaline phosphatase. A mutant that has a deletion in the alkaline phosphatase structural gene phoA produces the three proteins, but a newly discovered mutant phoB that has a defect in the expression of alkaline phosphatase fails to produce the three proteins. phoB mutants are shown here to be unable to make detectable amounts of alkaline phosphatase polypeptides, as measured by immunoprecipitins or acrylamide gel electropherograms. On the basis of these results we suggest a new model for the regulation of alkaline phosphatase biosynthesis. In this model, a ternary complex composed of phoB+ and phoR+ gene products and an internal metabolite functions as a positive control element to regulate the transcription of several cistrons coding for periplasmic proteins.
Full text
PDF


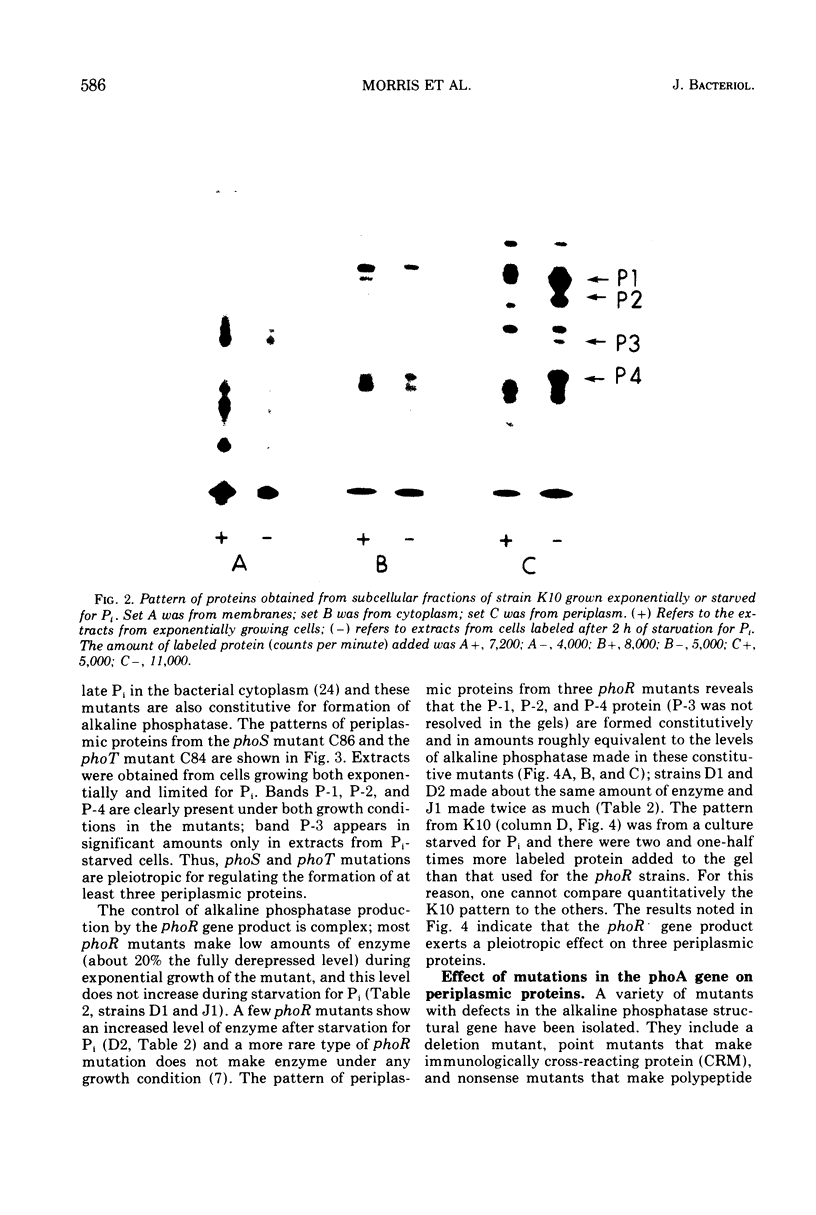




Images in this article
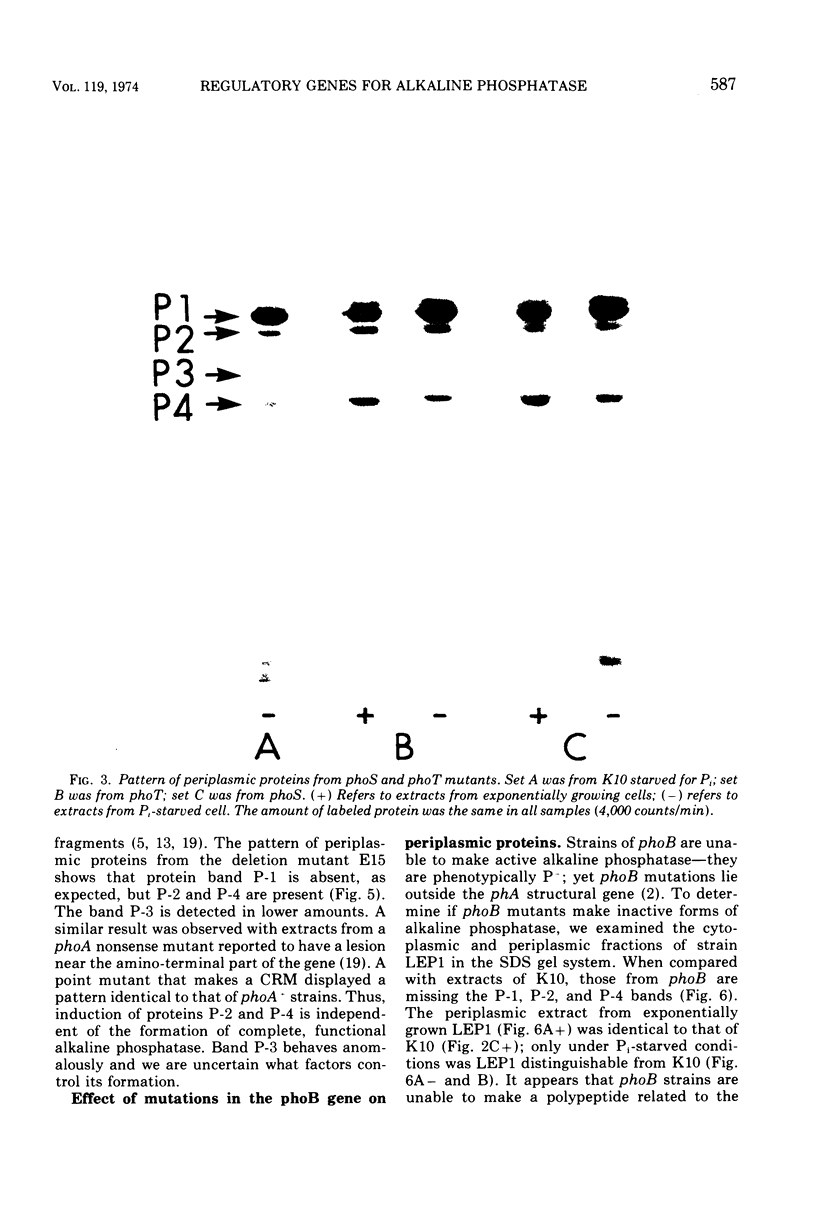
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Attias J., Schlesinger M. J., Schlesinger S. The effect of amino acid analogues on alkaline phosphatase formation in Escherichia coli K-12. IV. Substitution of canavanine for arginine. J Biol Chem. 1969 Jul 25;244(14):3810–3817. [PubMed] [Google Scholar]
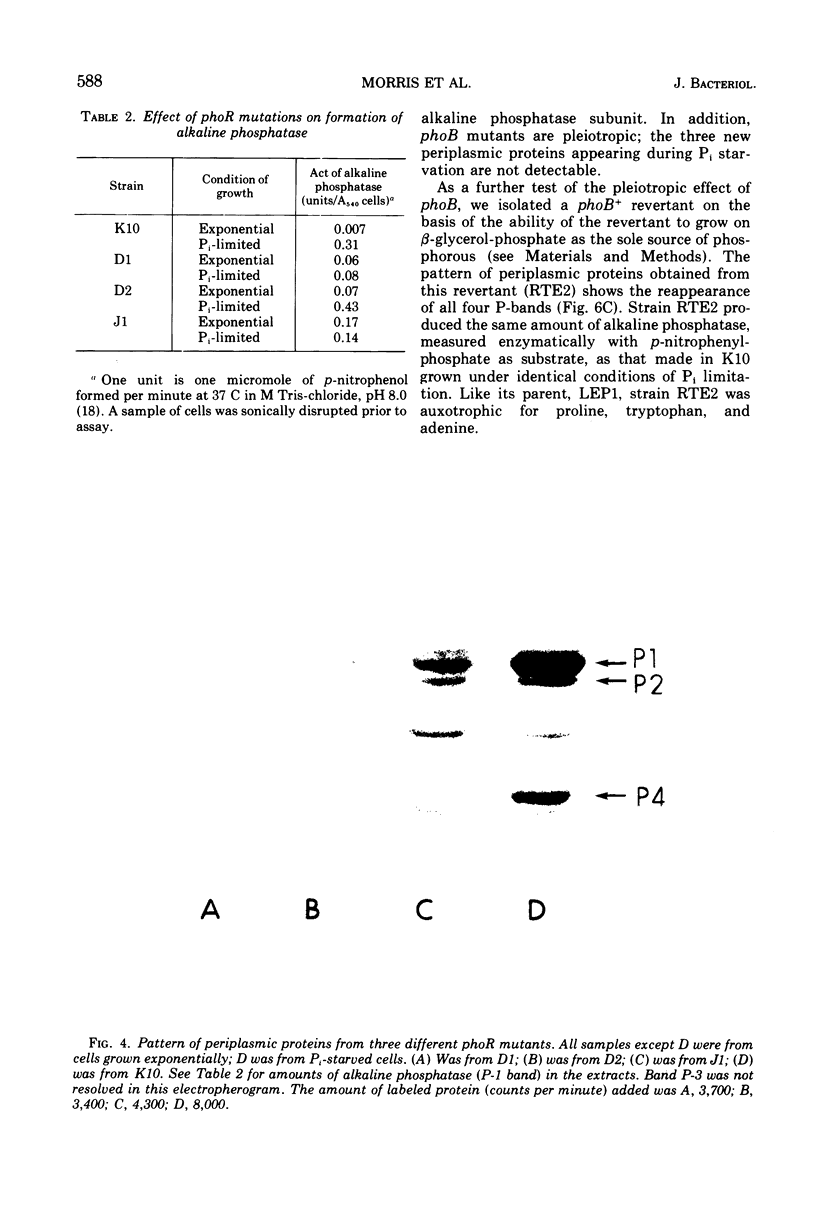
- Bracha M., Yagil E. A ne type of alkaline phosphatase-negative mutants in Escherichia coli K12. Mol Gen Genet. 1973 Mar 27;122(1):53–60. doi: 10.1007/BF00337973. [DOI] [PubMed] [Google Scholar]
- Cancedda R., Schlesinger M. J. Localization of polyribosomes containing alkaline phosphatase nascent polypeptides on membranes of Escherichia coli. J Bacteriol. 1974 Jan;117(1):290–301. doi: 10.1128/jb.117.1.290-301.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ECHOLS H., GAREN A., GAREN S., TORRIANI A. Genetic control of repression of alkaline phosphatase in E. coli. J Mol Biol. 1961 Aug;3:425–438. doi: 10.1016/s0022-2836(61)80055-7. [DOI] [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Genetic control of induction of alkaline phosphatase synthesis in E. coli. Proc Natl Acad Sci U S A. 1962 Aug;48:1398–1402. doi: 10.1073/pnas.48.8.1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., ECHOLS H. Properties of two regulating genes for alkaline phosphatase. J Bacteriol. 1962 Feb;83:297–300. doi: 10.1128/jb.83.2.297-300.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GAREN A., GAREN S. Genetic evidence on the nature of the repressor for alkaline phosphatase in E. coli. J Mol Biol. 1963 May;6:433–438. doi: 10.1016/s0022-2836(63)80054-6. [DOI] [PubMed] [Google Scholar]
- GAREN A., OTSUJI N. ISOLATION OF A PROTEIN SPECIFIED BY A REGULATOR GENE. J Mol Biol. 1964 Jun;8:841–852. doi: 10.1016/s0022-2836(64)80165-0. [DOI] [PubMed] [Google Scholar]
- Heppel L. A. Selective release of enzymes from bacteria. Science. 1967 Jun 16;156(3781):1451–1455. doi: 10.1126/science.156.3781.1451. [DOI] [PubMed] [Google Scholar]
- JACOB F., MONOD J. Genetic regulatory mechanisms in the synthesis of proteins. J Mol Biol. 1961 Jun;3:318–356. doi: 10.1016/s0022-2836(61)80072-7. [DOI] [PubMed] [Google Scholar]
- LEVINTHAL C. Genetic and chemical studies with alkaline phosphatase of E. coli. Brookhaven Symp Biol. 1959 Nov;12:76–85. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MALAMY M. H., HORECKER B. L. RELEASE OF ALKALINE PHOSPHATASE FROM CELLS OF ESCHERICHIA COLI UPON LYSOZYME SPHEROPLAST FORMATION. Biochemistry. 1964 Dec;3:1889–1893. doi: 10.1021/bi00900a017. [DOI] [PubMed] [Google Scholar]
- Morris H., Schlesinger M. J. Effects of proline analogues on the formation of alkaline phosphatase in Escherichia coli. J Bacteriol. 1972 Jul;111(1):203–210. doi: 10.1128/jb.111.1.203-210.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schlesinger M. J. Activation of a mutationally altered form of the Escherichia coli alkaline phosphatase by zinc. J Biol Chem. 1966 Jul 10;241(13):3181–3188. [PubMed] [Google Scholar]
- Schlesinger M. J. Formation of a defective alkaline phosphatase subunit by a mutant of Escherichia coli. J Biol Chem. 1967 Apr 10;242(7):1604–1611. [PubMed] [Google Scholar]
- Schlesinger S., Schlesinger M. J. The effect of amino acid analogues on alkaline phosphatase formation in Escherichia coli K-12. 3. Substitution of 2-methylhistidine for histidine. J Biol Chem. 1969 Jul 25;244(14):3803–3809. [PubMed] [Google Scholar]
- Studier F. W. Analysis of bacteriophage T7 early RNAs and proteins on slab gels. J Mol Biol. 1973 Sep 15;79(2):237–248. doi: 10.1016/0022-2836(73)90003-x. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Garen A. Fragments of alkaline phosphatase from nonsense mutants. I. Isolation and characterization of fragments from amber and ochre mutants. J Mol Biol. 1969 Nov 14;45(3):549–566. doi: 10.1016/0022-2836(69)90312-x. [DOI] [PubMed] [Google Scholar]
- TORRIANI A. Influence of inorganic phosphate in the formation of phosphatases by Escherichia coli. Biochim Biophys Acta. 1960 Mar 11;38:460–469. doi: 10.1016/0006-3002(60)91281-6. [DOI] [PubMed] [Google Scholar]
- Torriani A. Alkaline phosphatase subunits and their dimerization in vivo. J Bacteriol. 1968 Oct;96(4):1200–1207. doi: 10.1128/jb.96.4.1200-1207.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkins A. S. Physiological factors in the regulation of alkaline phosphatase synthesis in Escherichia coli. J Bacteriol. 1972 May;110(2):616–623. doi: 10.1128/jb.110.2.616-623.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willsky G. R., Bennett R. L., Malamy M. H. Inorganic phosphate transport in Escherichia coli: involvement of two genes which play a role in alkaline phosphatase regulation. J Bacteriol. 1973 Feb;113(2):529–539. doi: 10.1128/jb.113.2.529-539.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yagil E., Silberstein N. Decay of normal and 5-fluorouracil-substituted messenger ribonucleic acid of alkaline phosphatase in Escherichia coli. J Bacteriol. 1969 Dec;100(3):1364–1370. doi: 10.1128/jb.100.3.1364-1370.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]