Abstract
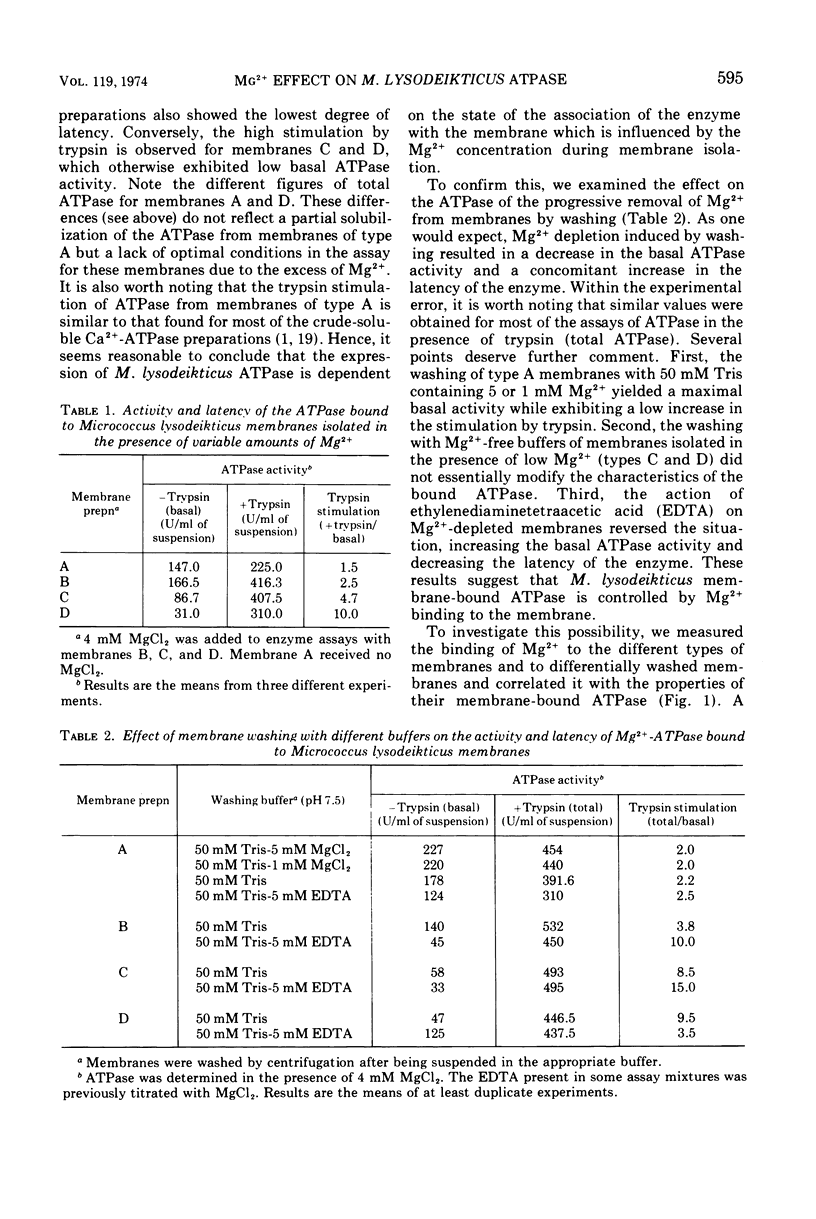
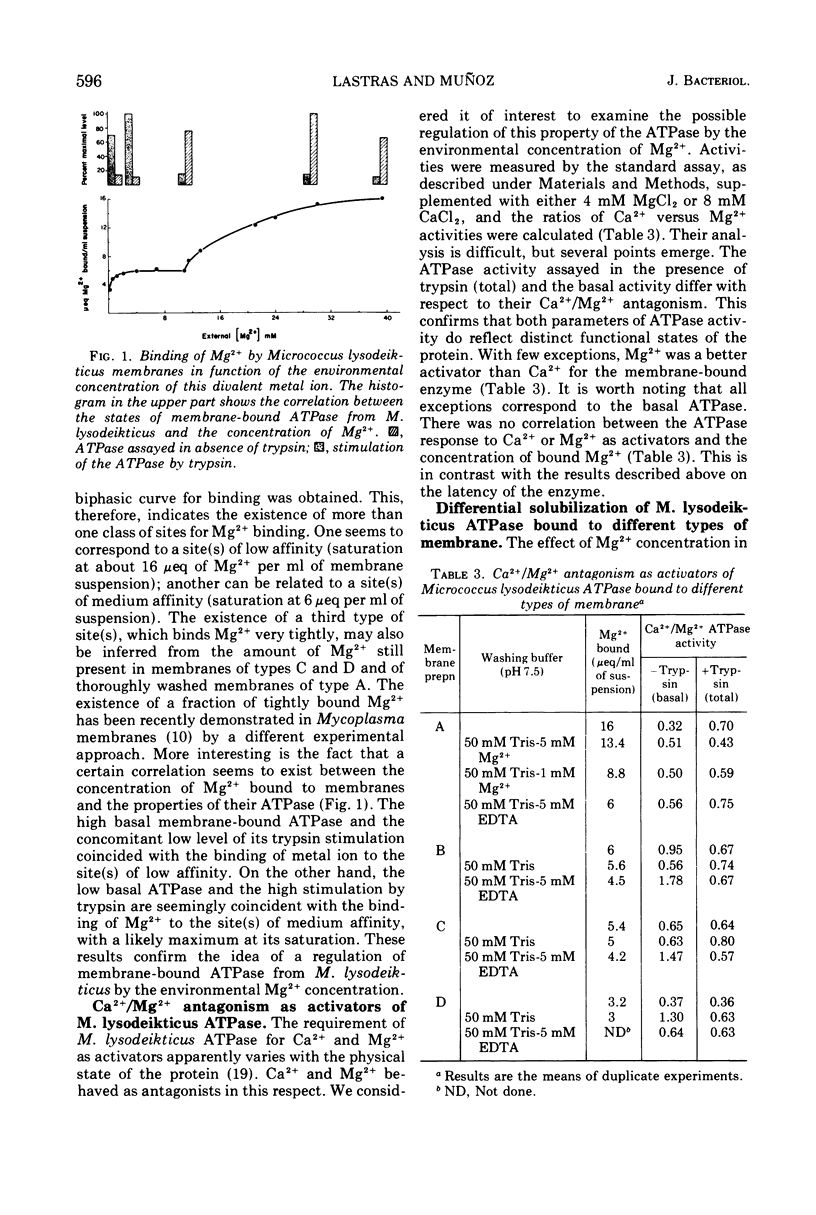
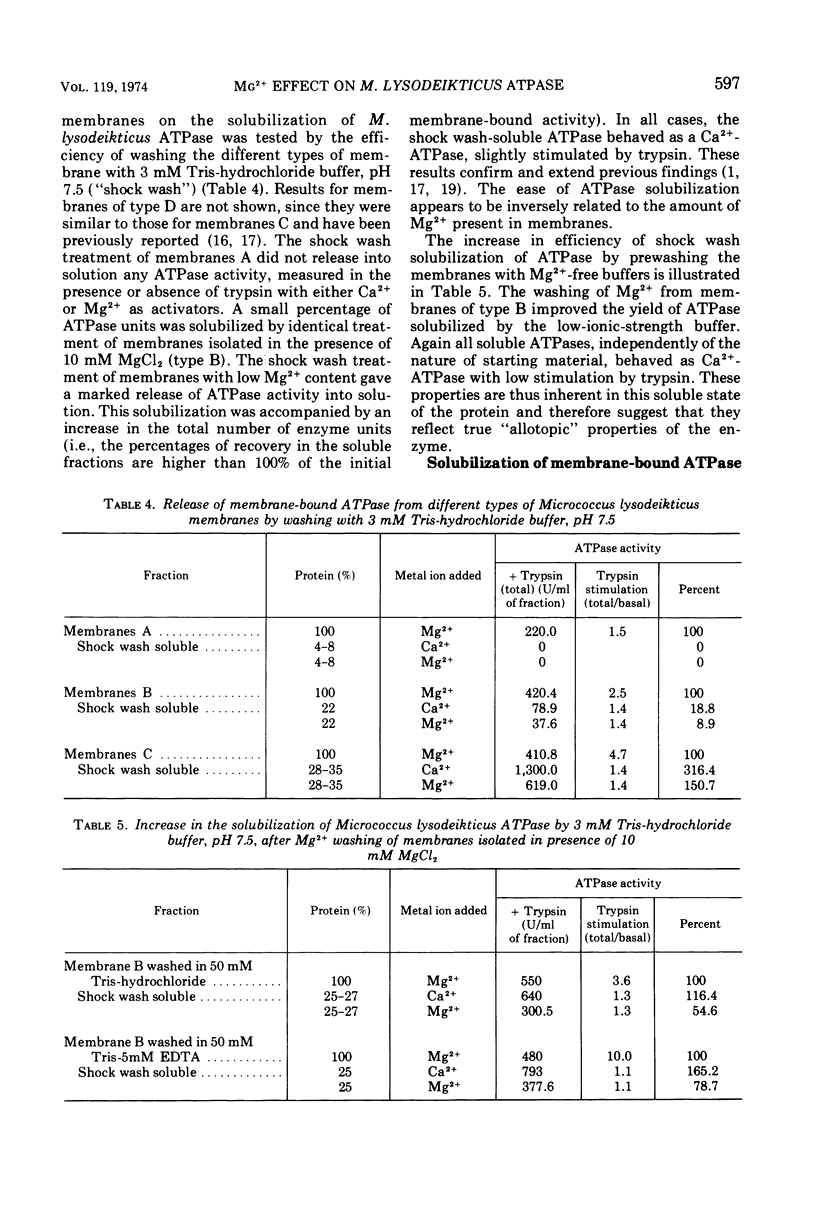
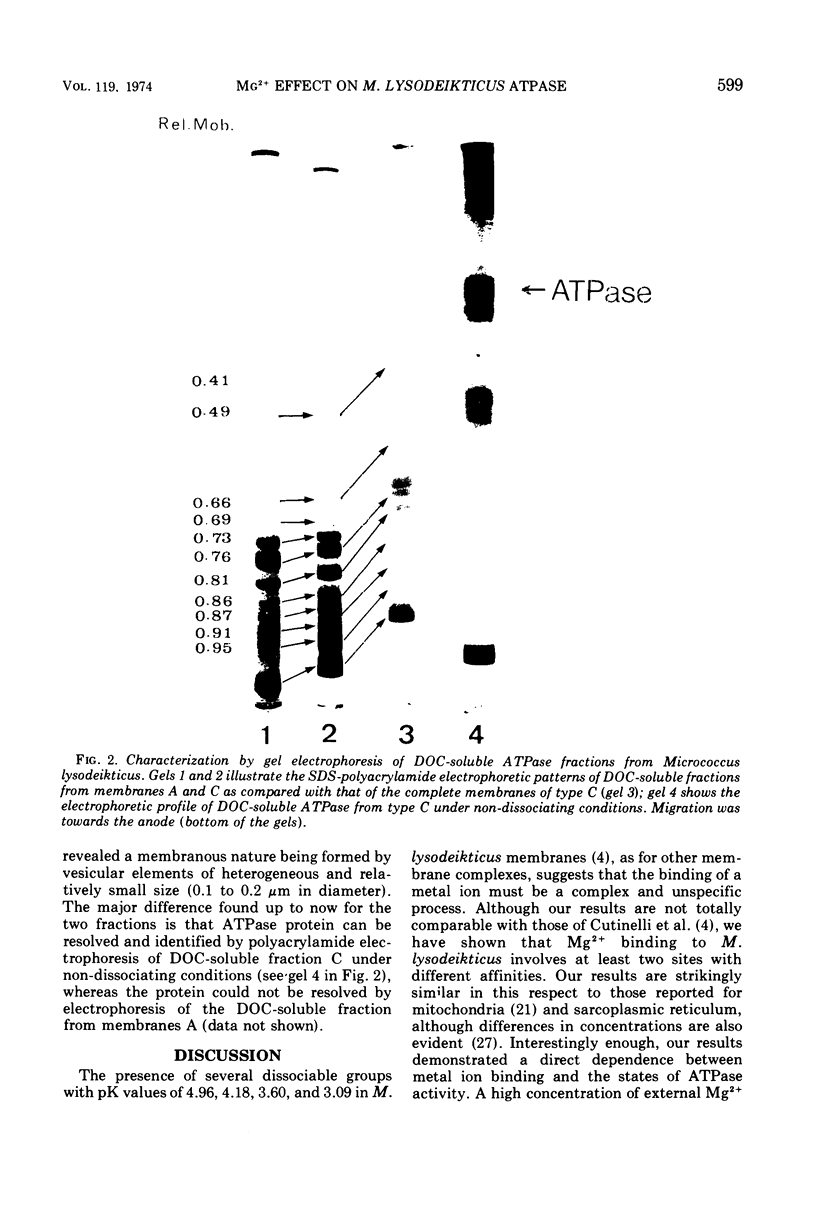
The latency of Micrococcus lysodeikticus membrane-bound Mg2+-adenosine triphosphatase (ATPase) is expressed by the ratio of its activity assayed in the presence of trypsin (“total”) versus the activity assayed in absence of the protease (“basal”). By isolating membranes in the presence of variable concentrations of Mg2+ (50 mM, 10 mM, or none) and by washing them with different Mg2+- and ethylenediaminetetraacetic acid-containing tris(hydroxymethyl)aminomethane-hydrochloride buffers (pH 7.5), we showed that the enzyme latency was dependent on the environmental concentration of this divalent metal ion. Mg2+ bound to at least two classes of sites. The binding of Mg2+ to low-affinity sites (saturation at approximately 40 mM external Mg2+) induced a high basal ATPase activity, whereas its binding to medium-affinity sites (saturation at about 2 mM Mg2+) correlated with low basal activity and a very high stimulation by trypsin. Membranes with tightly bound Mg2+ (high affinity?) revealed an intermediate behavior for the latency of M. lysodeikticus ATPase. The Mg2+/Ca2+ antagonism as activators of the membrane ATPase was not directly related to Mg2+ binding by the membranes. The efficiency of the ATPase release from M. lysodeikticus membrane by 3 mM tris(hydroxymethyl)aminomethane-hydrochloride buffer (pH 7.5) was inversely proportional to the concentration of external and/or bound Mg2+. Deoxycholate (DOC) (1%) solubilized the ATPase from all types of membrane. All the soluble ATPases behaved as Ca2+-ATPases, but the DOC-soluble fractions showed degrees of latency like those of the original membranes. The DOC-soluble ATPase preparation revealed a vesicular structure and complex protein patterns by sodium dodecyl sulfate gel electrophoresis. We propose that ATPase latency is modulated via a Mg2+-ATPase-membrane complex.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreu J. M., Albendea J. A., Munõz E. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Molecular properties of the purified enzyme unstimulated by trypsin. Eur J Biochem. 1973 Sep 3;37(3):505–515. doi: 10.1111/j.1432-1033.1973.tb03012.x. [DOI] [PubMed] [Google Scholar]
- Butler K. W., Dugas H., Smith I. C., Schneider H. Cation-induced organization changes in a lipid bilayer model membrane. Biochem Biophys Res Commun. 1970 Aug 24;40(4):770–776. doi: 10.1016/0006-291x(70)90969-1. [DOI] [PubMed] [Google Scholar]
- Carreira J., Leal J. A., Rojas M., Muñoz E. Membrane ATPase of Escherichia coli K 12. Selective solubilization of the enzyme and its stimulation by trypsin in the soluble and membrane-bound states. Biochim Biophys Acta. 1973 May 25;307(3):541–556. doi: 10.1016/0005-2736(73)90299-x. [DOI] [PubMed] [Google Scholar]
- Cutinelli C., Galdiero F., Tufano M. A. Cation-binding capacity of membranes isolated from Micrococcus lysodeikticus. J Bacteriol. 1969 Oct;100(1):123–127. doi: 10.1128/jb.100.1.123-127.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- De Vito E., Santomé J. A. Disc electrophoresis of proteins in the presence of sodium dodecyl sulphate. Experientia. 1966 Feb 15;22(2):124–125. doi: 10.1007/BF01900194. [DOI] [PubMed] [Google Scholar]
- Horvath C., Sovak M. Membrane coarctation by calcium as a regulator for bound enzymes. Biochim Biophys Acta. 1973 Apr 16;298(4):850–860. doi: 10.1016/0005-2736(73)90389-1. [DOI] [PubMed] [Google Scholar]
- Ishikawa S. Properties of an oxidative phosphorylation system reconstituted from coupling factors in Micrococcus lysodeikticus. J Biochem. 1970 Feb;67(2):297–312. doi: 10.1093/oxfordjournals.jbchem.a129253. [DOI] [PubMed] [Google Scholar]
- Kahane I., Ne'eman Z., Razin S. Divalent cations in native and reaggregated mycoplasma membranes. J Bacteriol. 1973 Feb;113(2):666–671. doi: 10.1128/jb.113.2.666-671.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lastras M., Muñoz E. Dependence on Mg(2+) for different states of the membrane-bound adenosine triphosphatase of Micrococcus lysodeikticus. FEBS Lett. 1971 Apr 12;14(1):69–72. doi: 10.1016/0014-5793(71)80277-6. [DOI] [PubMed] [Google Scholar]
- Lastras M., Muñoz E. Properties of the membrane-adenosine triphosphatase complex of Micrococcus lysodeikticus: Reversibility of the Mg(2+)-dependent states of the ATPase. FEBS Lett. 1972 Mar 15;21(2):109–112. doi: 10.1016/0014-5793(72)80115-7. [DOI] [PubMed] [Google Scholar]
- Lin D. C., Novel P. S. Control of photosynthesis by Mg 2+ . Arch Biochem Biophys. 1971 Aug;145(2):622–632. doi: 10.1016/s0003-9861(71)80022-x. [DOI] [PubMed] [Google Scholar]
- Loyter A., Christiansen R. O., Steensland H., Saltzgaber J., Racker E. Energy-linked ion translocation in submitochondrial particles. I. Ca++ accumulation in submitochondrial particles. J Biol Chem. 1969 Aug 25;244(16):4422–4427. [PubMed] [Google Scholar]
- Munoz E., Freer J. H., Ellar D. J., Salton M. R. Membrane-associated ATPase activity from Micrococcus lysodeikticus. Biochim Biophys Acta. 1968 Apr 29;150(3):531–533. doi: 10.1016/0005-2736(68)90156-9. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Nachbar M. S., Schor M. T., Salton M. R. Adenosinetriphosphatase of Micrococcus lysodeikticus: selective release and relationship to membrane structure. Biochem Biophys Res Commun. 1968 Aug 13;32(3):539–546. doi: 10.1016/0006-291x(68)90696-7. [DOI] [PubMed] [Google Scholar]
- Muñoz E., Salton M. R., Ng M. H., Schor M. T. Membrane adenosine triphosphatase of Micrococcus lysodeikticus. Purification, properties of the "soluble" enzyme and properties of the membrane-bound enzyme. Eur J Biochem. 1969 Feb;7(4):490–501. [PubMed] [Google Scholar]
- ORANGE M., RHEIN H. C. Microestimation of magnesium in body fluids. J Biol Chem. 1951 Mar;189(1):379–386. [PubMed] [Google Scholar]
- Reynafarje B., Lehninger A. L. High affinity and low affinity binding of Ca++ by rat liver mitochondria. J Biol Chem. 1969 Feb 25;244(4):584–593. [PubMed] [Google Scholar]
- Salton M. R., Freer J. H. Composition of the membranes isolated from several Gram-positive bacteria. Biochim Biophys Acta. 1965 Oct 18;107(3):531–538. doi: 10.1016/0304-4165(65)90197-2. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Schor M. T., Ng M. H. Internal localization of Micrococcus lysodeikticus membrane ATPase by iodination with 125 I. Biochim Biophys Acta. 1972 Dec 1;290(1):408–413. doi: 10.1016/0005-2736(72)90086-7. [DOI] [PubMed] [Google Scholar]
- Salton M. R., Schor M. T. Subunit structure and properties of two forms of adenosine triphosphatase released from Micrococcus lysodeikticus membranes. Biochem Biophys Res Commun. 1972 Oct 17;49(2):350–357. doi: 10.1016/0006-291x(72)90417-2. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Toury R. Etude de la fixation de faible affineitè du Ca2+ par les membranes externe et interne des mitochondries et par le rèticulum endoplasmique lisse et rugueux de foie de rat. Biochim Biophys Acta. 1973 May 25;307(3):607–612. doi: 10.1016/0005-2736(73)90305-2. [DOI] [PubMed] [Google Scholar]
- VAMBUTAS V. K., RACKER E. PARTIAL RESOLUTION OF THE ENZYMES CATALYZINE PHOTOPHOSPHORYLATION. I. STIMULATION OF PHOTOPHOSPHORYLATION BY A PREPARATION OF A LATENT, CA++- DEPENDENT ADENOSINE TRIPHOSPHATASE FROM CHLOROPLASTS. J Biol Chem. 1965 Jun;240:2660–2667. [PubMed] [Google Scholar]




