Abstract
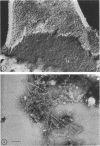
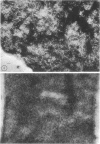
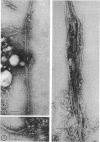
The cell wall of the yeast form of Histoplasma farciminosum contains 13.2% β-1,3-glucan, 1.0% galactomannan, and 25.8% chitin, whereas the cell wall of mycelial form has 21.8, 4.5, and 40%, respectively, for the same polymers. Also, the cell wall of the yeast form contains α-1,3-glucan (13.5%) and an unidentified polymer (21.5%). Chitin, one of the structural polymers of both yeast and mycelial cell walls, is identified as thin isolated fibers (4 nm wide) or in thick bundles (50 nm wide) of fibers. β-(1-3)-Glucan is also found as thin isolated fibers indistinguishable from isolated fibers of chitin. Fibers 14 nm wide and resembling α-(1-3)-glucan fibers of other fungi are found in the yeast form. The results reported here do not give support to the proposal for a different taxonomic classification.
Full text
PDF

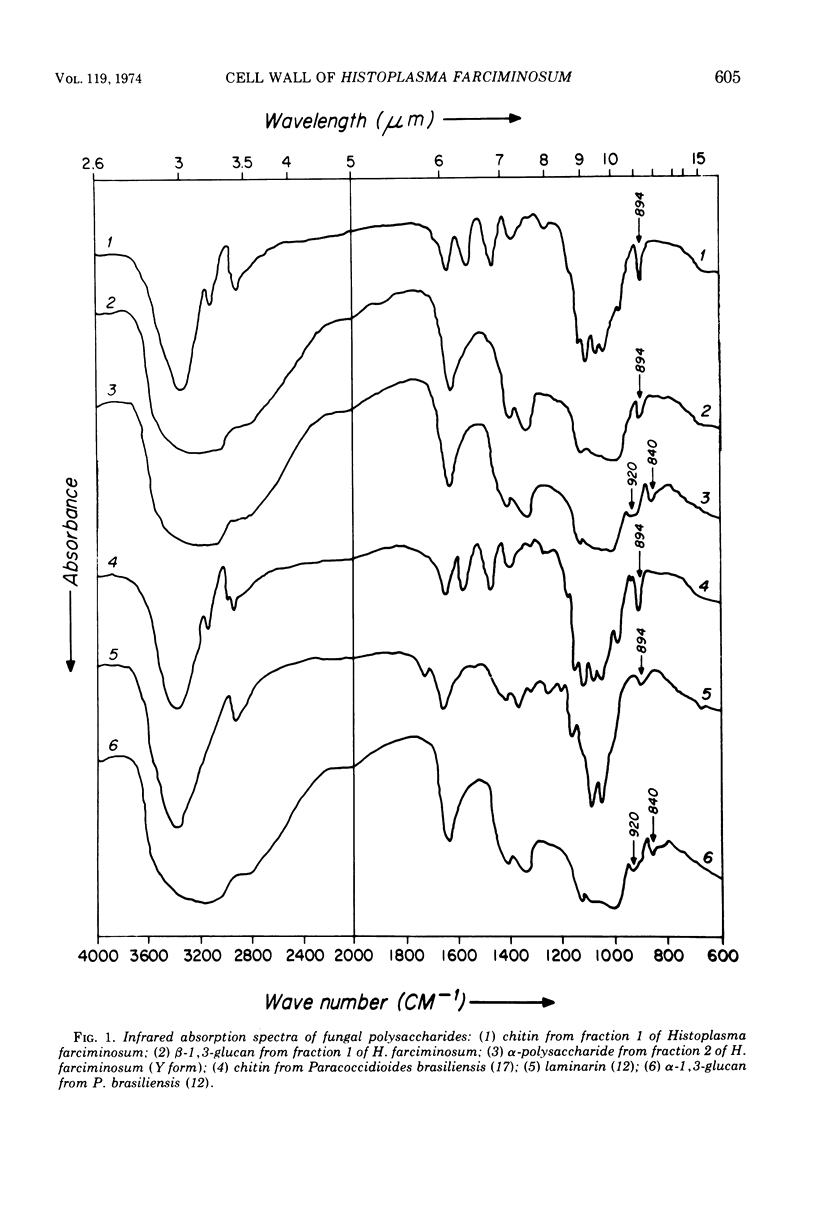

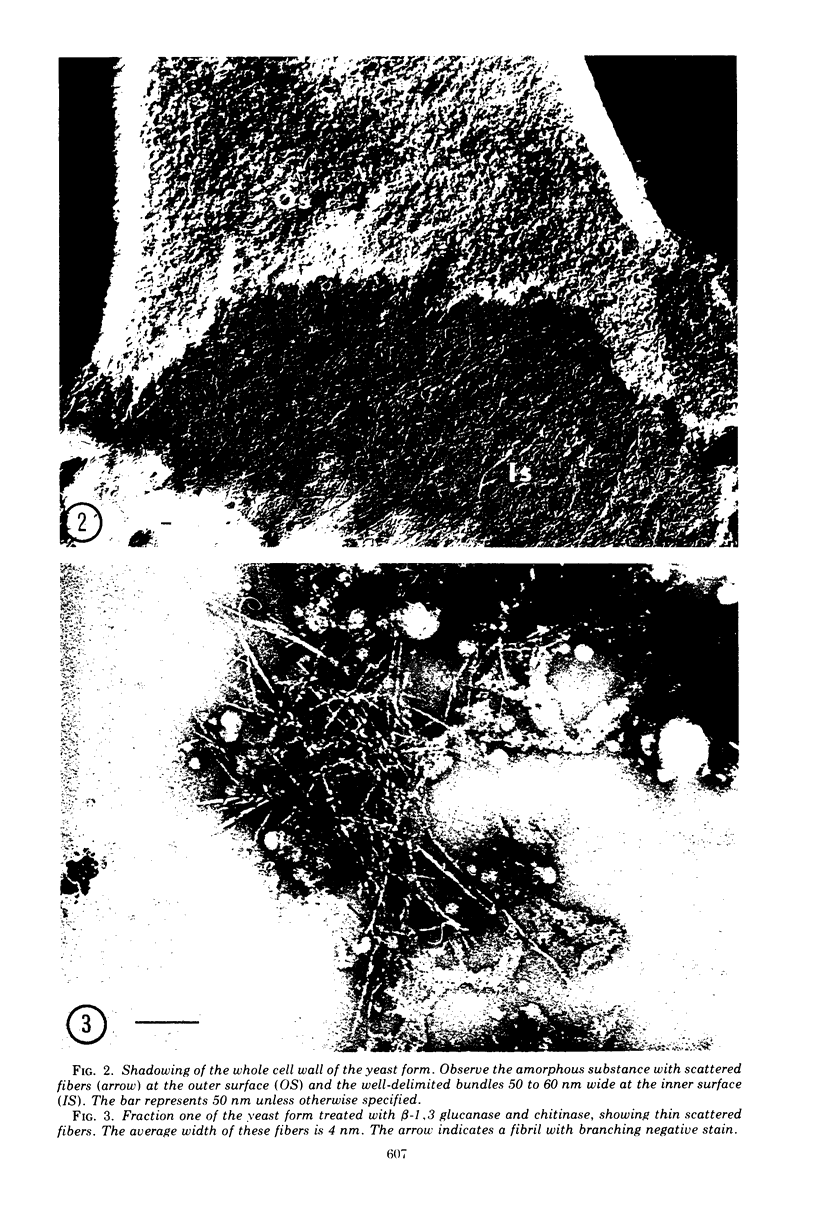

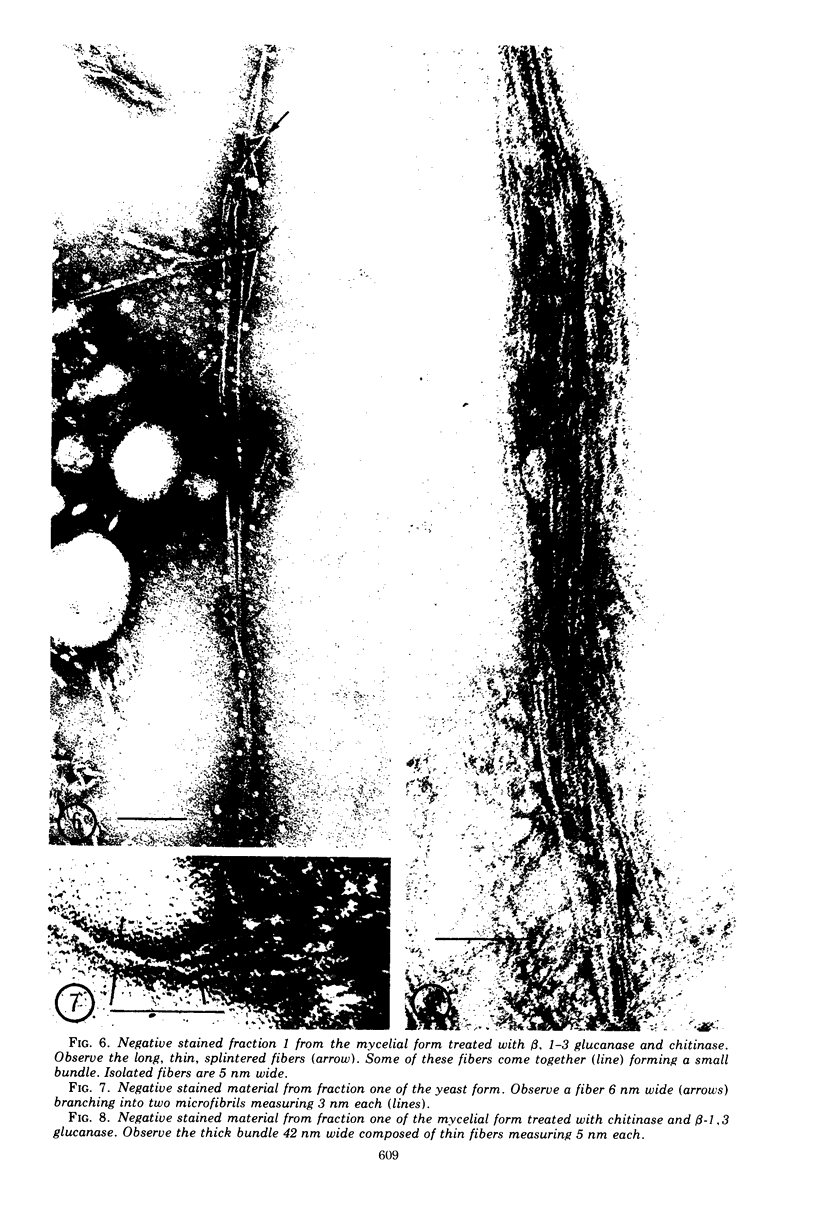


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartnicki-Garcia S. Cell wall chemistry, morphogenesis, and taxonomy of fungi. Annu Rev Microbiol. 1968;22:87–108. doi: 10.1146/annurev.mi.22.100168.000511. [DOI] [PubMed] [Google Scholar]
- Bartnicki-Garcia S., Lippman E. Fungal morphogenesis: cell wall construction in Mucor rouxii. Science. 1969 Jul 18;165(3890):302–304. doi: 10.1126/science.165.3890.302. [DOI] [PubMed] [Google Scholar]
- Cabib E., Bowers B. Chitin and yeast budding. Localization of chitin in yeast bud scars. J Biol Chem. 1971 Jan 10;246(1):152–159. [PubMed] [Google Scholar]
- Carbonell L. M., Kanetsuna F., Gil F. Chemical morphology of glucan and chitin in the cell wall of the yeast phase of Paracoccidioides brasiliensis. J Bacteriol. 1970 Feb;101(2):636–642. doi: 10.1128/jb.101.2.636-642.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E., Hamilton J. G., Harkin J. C. Comparative study of the cell walls of the yeastlike and mycelial phases of Histoplasma capsulatum. J Bacteriol. 1967 Aug;94(2):466–474. doi: 10.1128/jb.94.2.466-474.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domer J. E. Monosaccharide and chitin content of cell walls of Histoplasma capsulatum and Blastomyces dermatitidis. J Bacteriol. 1971 Sep;107(3):870–877. doi: 10.1128/jb.107.3.870-877.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasegawa S., Nordin J. H. Enzymes that hydrolyze fungal cell wall polysaccharides. I. Purification and properties of an endo-alpha-D-(1-3)-glucanase from Trichoderma. J Biol Chem. 1969 Oct 25;244(20):5460–5470. [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M., Azuma I., Yamamura Y. Biochemical studies on the thermal dimorphism of Paracoccidioides brasiliensis. J Bacteriol. 1972 Apr;110(1):208–218. doi: 10.1128/jb.110.1.208-218.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M. Cell wall composition of the yeastlike and mycelial forms of Blastomyces dermatitidis. J Bacteriol. 1971 Jun;106(3):946–948. doi: 10.1128/jb.106.3.946-948.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M. Cell wall glucans of the yeast and mycelial forms of Paracoccidioides brasiliensis. J Bacteriol. 1970 Mar;101(3):675–680. doi: 10.1128/jb.101.3.675-680.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanetsuna F., Carbonell L. M., Moreno R. E., Rodriguez J. Cell wall composition of the yeast and mycelial forms of Paracoccidioides brasiliensis. J Bacteriol. 1969 Mar;97(3):1036–1041. doi: 10.1128/jb.97.3.1036-1041.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi G. S., Guiliacci P. L. Cell wall studies of Histoplasma capsulatum. Sabouraudia. 1967 Feb;5(3):180–188. [PubMed] [Google Scholar]
- Moreno R. E., Kanetsuna F., Carbonell L. M. Isolation of chitin and glucan from the cell wall of the yeast form of Paracoccidioides brasiliensis. Arch Biochem Biophys. 1969 Mar;130(1):212–217. doi: 10.1016/0003-9861(69)90026-5. [DOI] [PubMed] [Google Scholar]
- Pine L., Boone C. J. Cell wall composition and serological reactivity of Histoplasma capsulatum serotypes and related species. J Bacteriol. 1968 Sep;96(3):789–798. doi: 10.1128/jb.96.3.789-798.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REESE E. T., MANDELS M. Beta-D-1, 3 Glucanases in fungi. Can J Microbiol. 1959 Apr;5(2):173–185. doi: 10.1139/m59-022. [DOI] [PubMed] [Google Scholar]
- Stagg C. M., Feather M. S. The characterization of a chitin-associated D-glucan from the cell walls of Aspergillus niger. Biochim Biophys Acta. 1973 Aug 17;320(1):64–72. doi: 10.1016/0304-4165(73)90166-9. [DOI] [PubMed] [Google Scholar]
- WHITE J. W., Jr, SUBERS M. H. A glucose oxidase reagent for maltase assay. Anal Biochem. 1961 Aug;2:380–384. doi: 10.1016/0003-2697(61)90011-2. [DOI] [PubMed] [Google Scholar]