Abstract
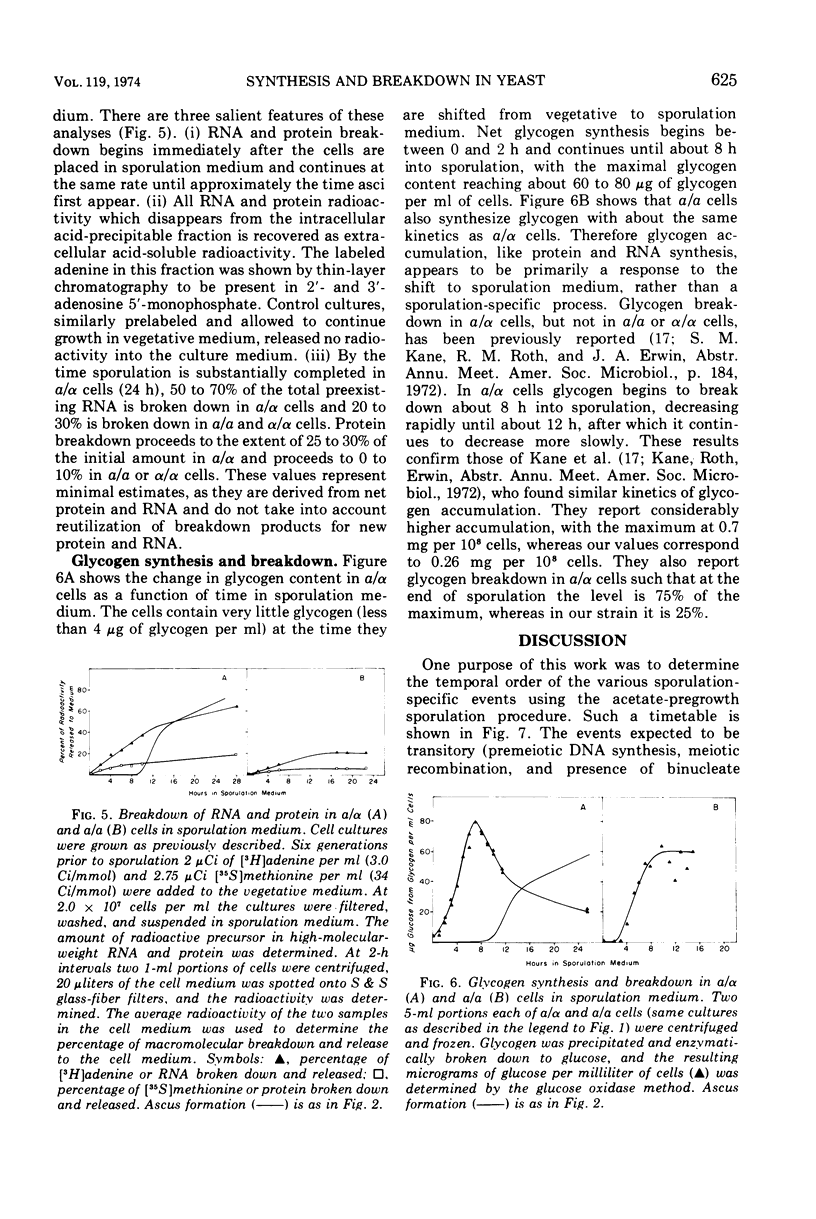
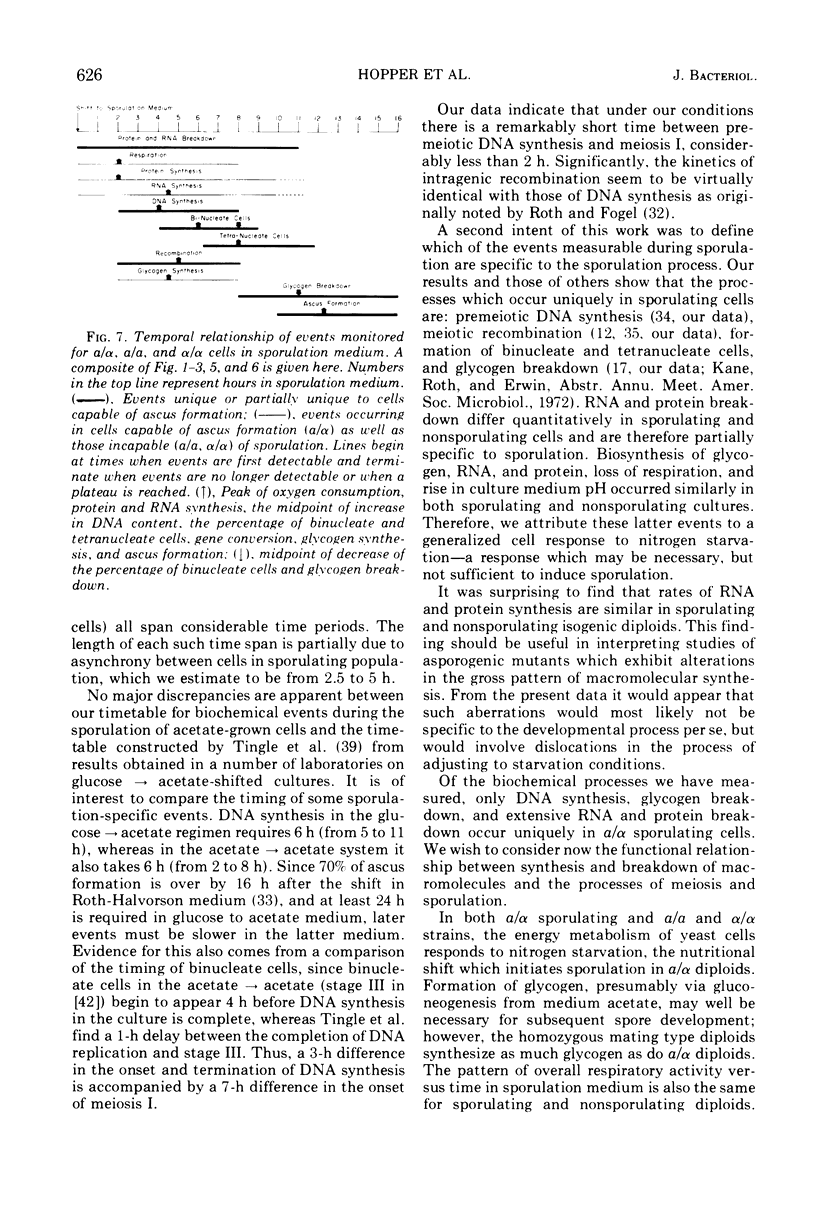
The time course of synthesis and breakdown of various macromolecules has been compared for sporulating (a/α) and nonsporulating (a/a and α/α) yeast cells transferred to potassium acetate sporulation medium. Both types of cells incorporate label into ribonucleic acid and protein. The gel electrophoresis patterns of proteins synthesized in sporulation medium are identical for sporulating and nonsporulating diploids; both are different from electropherograms of vegetative cells. Sporulating and nonsporulating strains differ with respect to deoxyribonucleic acid synthesis; no deoxyribonucleic acid is synthesized in the latter case, whereas the deoxyribonucleic acid complement is doubled in the former. Glycogen breakdown occurs only in sporulating strains. Breakdown of preexisting vegetative ribonucleic acid and protein molecules occurs much more extensively in sporulating than in nonsporulating cells. A timetable of these data is presented.
Full text
PDF


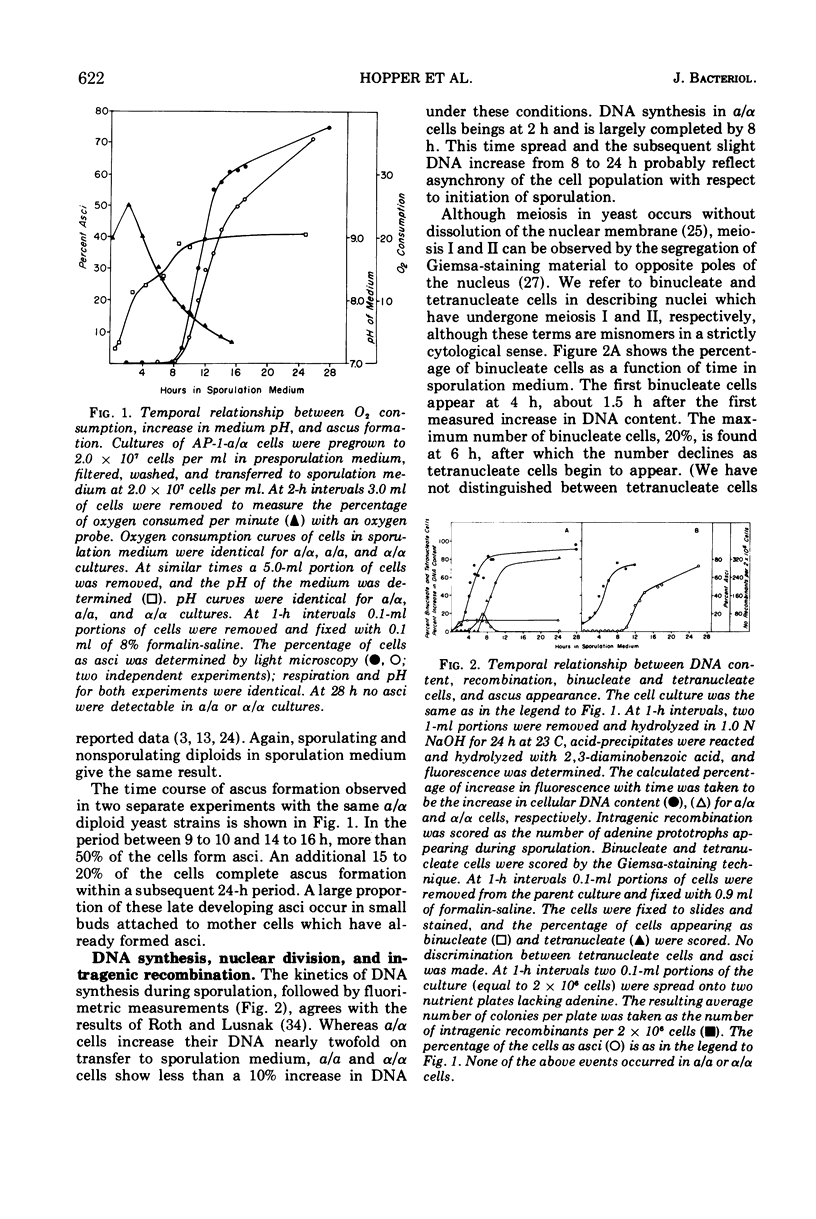


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen A. W., Miller J. J. Proteolytic activity of intact yeast cells during sporulation. Can J Microbiol. 1968 Sep;14(9):957–963. doi: 10.1139/m68-159. [DOI] [PubMed] [Google Scholar]
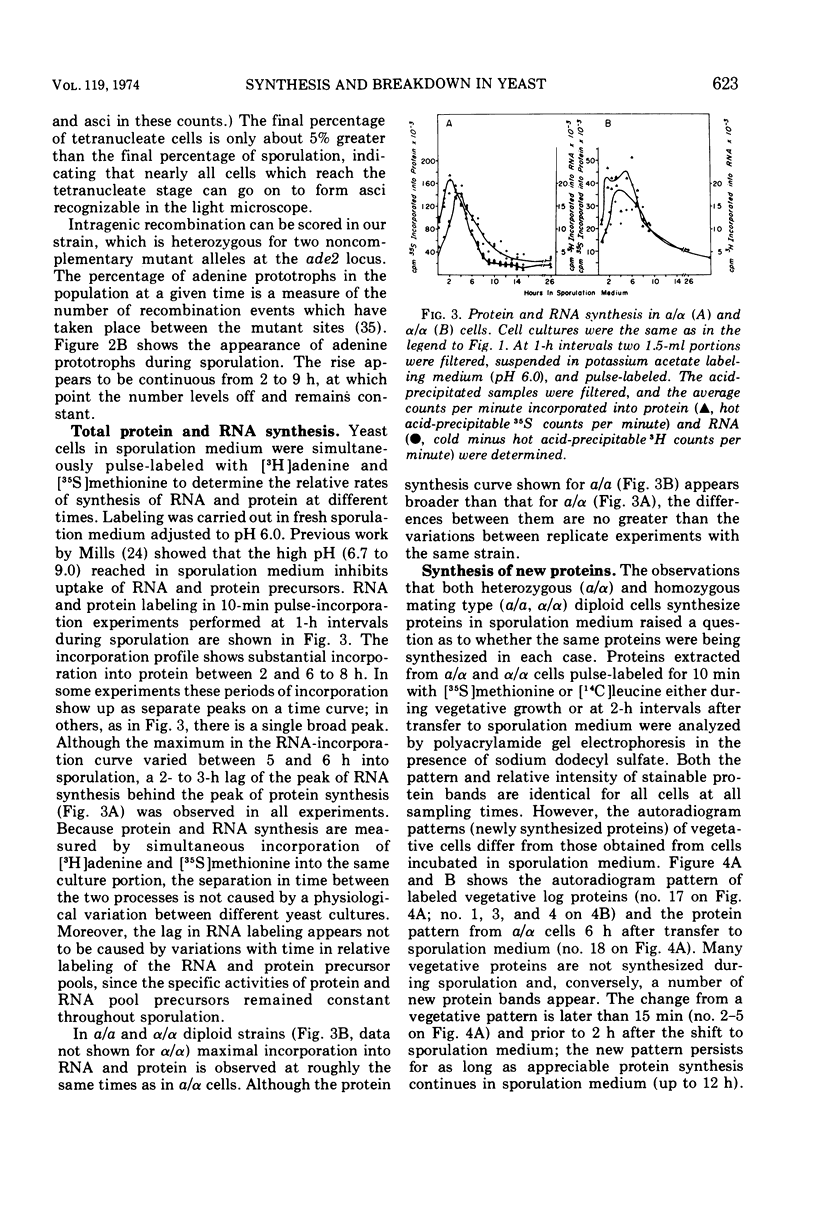
- Dickinson H. G., Heslop-Harrison J. The ribosome cycle, nucleoli, and cytoplasmic nucleoloids in the meiocytes of Lilium. Protoplasma. 1970;69(2):189–200. doi: 10.1007/BF01280721. [DOI] [PubMed] [Google Scholar]
- EPHRUSSI B., HOTTINGUER H. On an unstable cell state in yeast. Cold Spring Harb Symp Quant Biol. 1951;16:75–85. doi: 10.1101/sqb.1951.016.01.007. [DOI] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E., Arnaud M., Halvorson H. O. Acetate utilization and macromolecular synthesis during sporulation of yeast. J Bacteriol. 1969 Oct;100(1):180–186. doi: 10.1128/jb.100.1.180-186.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito M. S., Esposito R. E. The genetic control of sporulation in Saccharomyces. I. The isolation of temperature-sensitive sporulation-deficient mutants. Genetics. 1969 Jan;61(1):79–89. doi: 10.1093/genetics/61.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito R. E., Frink N., Bernstein P., Esposito M. S. The genetic control of sporulation in Saccharomyces. II. Dominance and complementation of mutants of meiosis and spore formation. Mol Gen Genet. 1972;114(3):241–248. doi: 10.1007/BF01788893. [DOI] [PubMed] [Google Scholar]
- Fogel S., Mortimer R. K. Recombination in yeast. Annu Rev Genet. 1971;5:219–236. doi: 10.1146/annurev.ge.05.120171.001251. [DOI] [PubMed] [Google Scholar]
- Friis J., Roman H. The effect of the mating-type alleles on intragenic recombination in yeast. Genetics. 1968 May;59(1):33–36. doi: 10.1093/genetics/59.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galzy P., Bizeau C. Action de l'acide acétique sur la sporulation de Saccharomyces cerevisiae Hansen. C R Seances Soc Biol Fil. 1966;160(1):176–178. [PubMed] [Google Scholar]
- Goldberg A. L. Effects of protease inhibitors on protein breakdown and enzyme induction in starving Escherichia coli. Nat New Biol. 1971 Nov 10;234(45):51–52. doi: 10.1038/newbio234051a0. [DOI] [PubMed] [Google Scholar]
- Hartwell L. H. Macromolecule synthesis in temperature-sensitive mutants of yeast. J Bacteriol. 1967 May;93(5):1662–1670. doi: 10.1128/jb.93.5.1662-1670.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartwell L. H. Periodic density fluctuation during the yeast cell cycle and the selection of synchronous cultures. J Bacteriol. 1970 Dec;104(3):1280–1285. doi: 10.1128/jb.104.3.1280-1285.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KISSANE J. M., ROBINS E. The fluorometric measurement of deoxyribonucleic acid in animal tissues with special reference to the central nervous system. J Biol Chem. 1958 Jul;233(1):184–188. [PubMed] [Google Scholar]
- Kane S. M., Roth R. Carbohydrate metabolism during ascospore development in yeast. J Bacteriol. 1974 Apr;118(1):8–14. doi: 10.1128/jb.118.1.8-14.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lee E. Y., Carter J. H., Nielsen L. D., Fischer E. H. Purification and properties of yeast amylo-1,6-glucosidase--oligo-1,4 leads to 1,4-glucantransferase. Biochemistry. 1970 May 26;9(11):2347–2355. doi: 10.1021/bi00813a019. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. The intracellular turnover of protein and nucleic acids and its role in biochemical differentiation. Bacteriol Rev. 1960 Sep;24(3):289–308. doi: 10.1128/br.24.3.289-308.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MILLER J. J., HOFFMANN-OSTENHOF C. SPORE FORMATION AND GERMINATION IN SACCHAROMYCES. Z Allg Mikrobiol. 1964;4:273–294. doi: 10.1002/jobm.3630040404. [DOI] [PubMed] [Google Scholar]
- Mills D. Effect of pH on adenine and amino acid uptake during sporulation in Saccharomyces cerevisiae. J Bacteriol. 1972 Oct;112(1):519–526. doi: 10.1128/jb.112.1.519-526.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P. B., Rapport E. Spindles, spindle plaques, and meiosis in the yeast Saccharomyces cerevisiae (Hansen). J Cell Biol. 1971 Aug;50(2):344–361. doi: 10.1083/jcb.50.2.344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mortimer R. K., Hawthorne D. C. Genetic Mapping in Saccharomyces IV. Mapping of Temperature-Sensitive Genes and Use of Disomic Strains in Localizing Genes. Genetics. 1973 May;74(1):33–54. doi: 10.1093/genetics/74.1.33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROMAN H., JACOB F. A comparison of spontaneous and ultraviolet-induced allelic recombination with reference to the recombination of outside markers. Cold Spring Harb Symp Quant Biol. 1958;23:155–160. doi: 10.1101/sqb.1958.023.01.019. [DOI] [PubMed] [Google Scholar]
- Robinow C. F., Marak J. A fiber apparatus in the nucleus of the yeast cell. J Cell Biol. 1966 Apr;29(1):129–151. doi: 10.1083/jcb.29.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman H., Sands S. M. Heterogeneity of Clones of Saccharomyces Derived from Haploid Ascospores. Proc Natl Acad Sci U S A. 1953 Mar;39(3):171–179. doi: 10.1073/pnas.39.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R. Carbohydrate accumulation during the sporulation of yeast. J Bacteriol. 1970 Jan;101(1):53–57. doi: 10.1128/jb.101.1.53-57.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Fogel S. A system selective for yeast mutants deficient in meiotic recombination. Mol Gen Genet. 1971;112(4):295–305. doi: 10.1007/BF00334431. [DOI] [PubMed] [Google Scholar]
- Roth R., Halvorson H. O. Sporulation of yeast harvested during logarithmic growth. J Bacteriol. 1969 May;98(2):831–832. doi: 10.1128/jb.98.2.831-832.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roth R., Lusnak K. DNA synthesis during yeast sporulation: genetic control of an early developmental event. Science. 1970 Apr 24;168(3930):493–494. doi: 10.1126/science.168.3930.493. [DOI] [PubMed] [Google Scholar]
- SHERMAN F., ROMAN H. Evidence for two types of allelic recombination in yeast. Genetics. 1963 Feb;48:255–261. doi: 10.1093/genetics/48.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SHERMAN F., SLONIMSKI P. P. RESPIRATION-DEFICIENT MUTANTS OF YEAST. II. BIOCHEMISTRY. Biochim Biophys Acta. 1964 Jul 15;90:1–15. doi: 10.1016/0304-4165(64)90113-8. [DOI] [PubMed] [Google Scholar]
- Simchen G., Salts Y., Piñon R. Sensitivity of meiotic yeast cells to ultraviolet light. Genetics. 1973 Apr;73(4):531–541. doi: 10.1093/genetics/73.4.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon M. N., Studier F. W. Physical mapping of the early region of bacteriophage T7 DNA. J Mol Biol. 1973 Sep 15;79(2):249–265. doi: 10.1016/0022-2836(73)90004-1. [DOI] [PubMed] [Google Scholar]