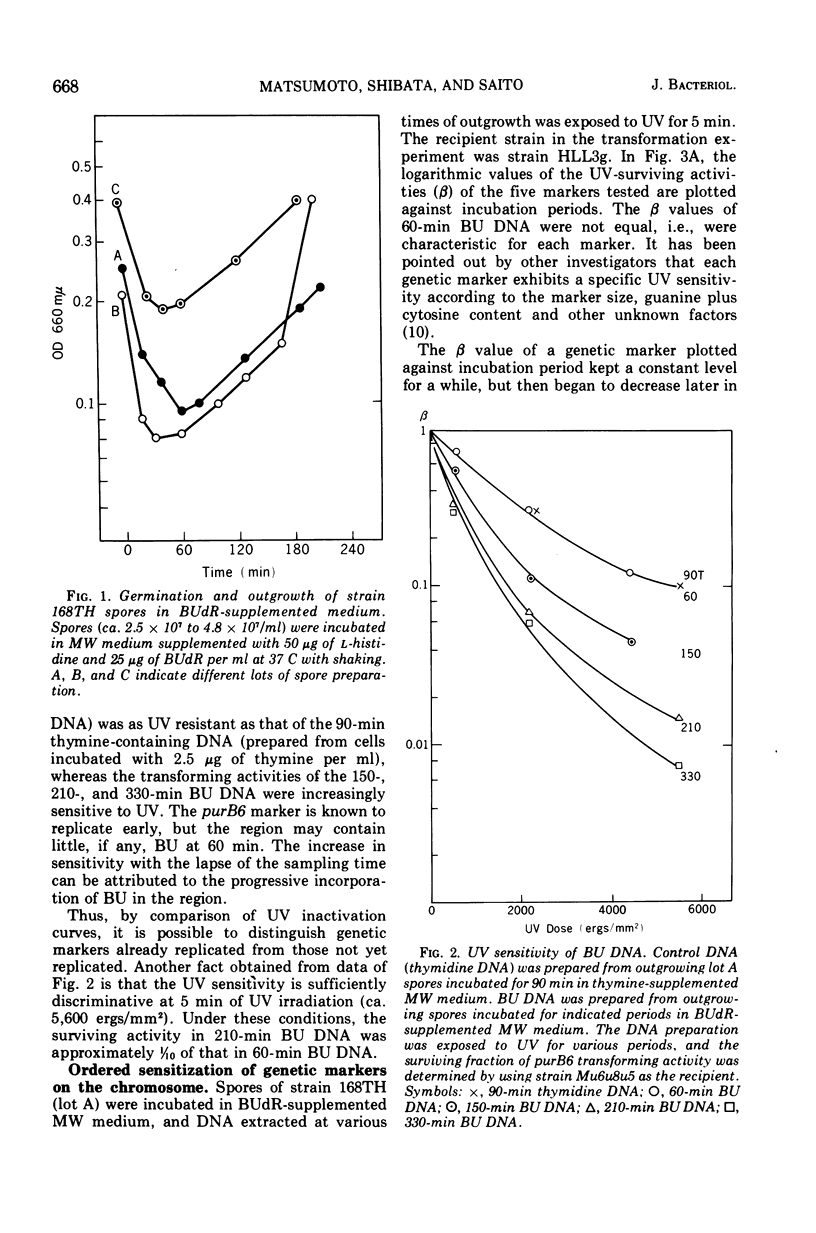
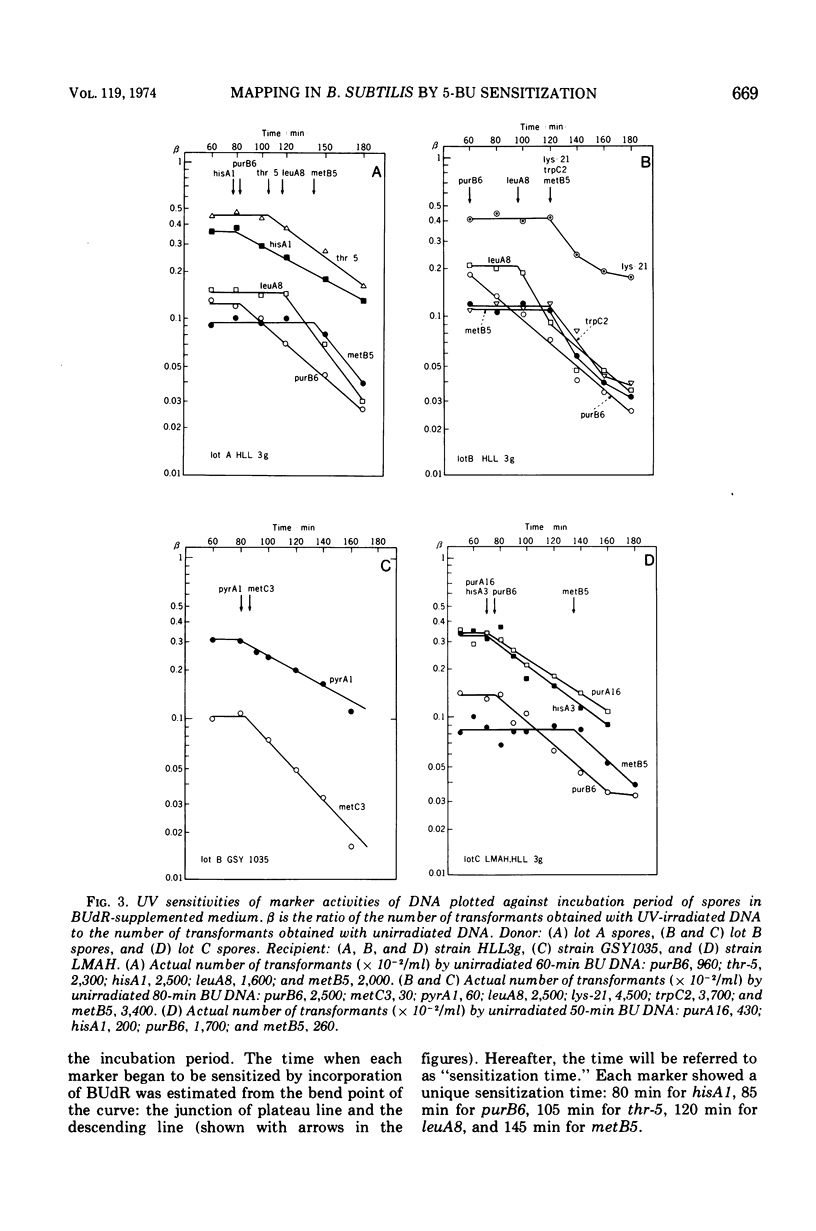
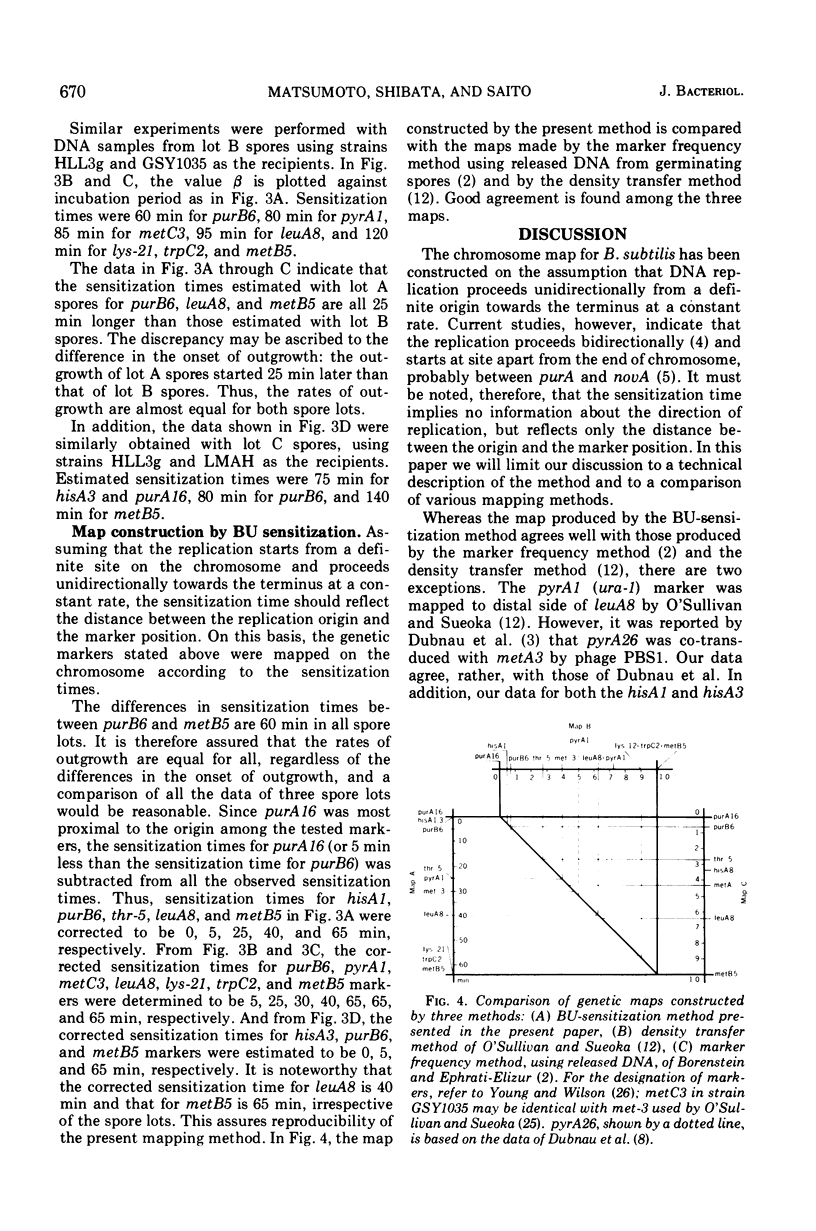
Abstract
A new method for chromosome mapping of Bacillus subtilis Marburg is presented which is based on the sensitization to ultraviolet irradiation of transforming deoxyribonucleic acid that has incorporated 5-bromouracil instead of thymine. Deoxyribonucleic acid was extracted at intervals from the outgrowing spores of a thymine-requiring mutant incubated with 5-bromodeoxyuridine and subjected to a definite dose of ultraviolet irradiation. The residual activities of various genetic markers were assayed by transformation. The marker activity of deoxyribonucleic acid that had incorporated 5-bromodeoxyuridine was approximately 10 times as sensitive to ultraviolet irradiation as that of normal deoxyribonucleic acid. The markers proximal to the replication origin were sensitized at earlier times of outgrowth than distal markers. The chromosome replication in outgrowing spores was sufficiently synchronous and allowed the definite determination of when a marker became sensitized by incorporation of 5-bromodeoxyuridine. The time, designated “sensitization time,” was estimated by plotting the logarithmic values of relative residual activities versus incubation times. The map constructed with sensitization times as a measurement showed good agreement with those constructed by other methods. The replication of the chromosome under the described conditions appeared to occur in the following marker order: (purA, hisA)-(purB)-(thr, pyrA)-(metC)-(leuA)-(lys, trpC, metB).
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop R. J., Sueoka N. 5-Bromouracil-tolerant mutants of Bacillus subtilis. J Bacteriol. 1972 Nov;112(2):870–876. doi: 10.1128/jb.112.2.870-876.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borenstein S., Ephrati-Elizur E. Spontaneous release of DNA in sequential genetic order by Bacillus subtilis. J Mol Biol. 1969 Oct 14;45(1):137–152. doi: 10.1016/0022-2836(69)90216-2. [DOI] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Gyurasits E. B., Wake R. G. Bidirectional chromosome replication in Bacillus subtilis. J Mol Biol. 1973 Jan;73(1):55–63. doi: 10.1016/0022-2836(73)90158-7. [DOI] [PubMed] [Google Scholar]
- Hara H., Yoshikawa H. Asymmetric bidirectional replication of Bacillus subtilis chromosome. Nat New Biol. 1973 Aug 15;244(137):200–203. doi: 10.1038/newbio244200a0. [DOI] [PubMed] [Google Scholar]
- Hotz G., Walser R. On the mechnaism of radiosensitization by 5-bromouracil. The occurrence of DNA strand breaks in U.V.-irradiated phage T4 as influenced by cysteamine. Photochem Photobiol. 1970 Sep;12(3):207–218. doi: 10.1111/j.1751-1097.1970.tb06052.x. [DOI] [PubMed] [Google Scholar]
- Hutchinson F., Hales H. B. Mechanism of the sensitization of bacterial transforming DNA to ultraviolet light by the incorporation of 5-bromouracil. J Mol Biol. 1970 May 28;50(1):59–69. doi: 10.1016/0022-2836(70)90103-8. [DOI] [PubMed] [Google Scholar]
- Lion M. B. Search for a mechanism for the increased sensitivity of 5-bromouracil-substituted DNA to ultraviolet radiation. II. Single-strand breaks in the DNA of irradiated 5-bromouracil-substituted T3 coliphage. Biochim Biophys Acta. 1970 May 21;209(1):24–33. doi: 10.1016/0005-2787(70)90657-x. [DOI] [PubMed] [Google Scholar]
- Munakata N., Saito H., Ikeda Y. Inactivation of transforming DNA by ultraviolet irradiation. Mutat Res. 1966 Apr;3(2):93–100. doi: 10.1016/0027-5107(66)90022-4. [DOI] [PubMed] [Google Scholar]
- Nagley P., Wake R. G. Effect of 5-bromouracil on the pattern of deoxyribonucleic acid replication in germinating Bacillus subtilis spores. J Mol Biol. 1969 Aug 14;43(3):619–630. doi: 10.1016/0022-2836(69)90363-5. [DOI] [PubMed] [Google Scholar]
- O'Sullivan A., Sueoka N. Sequential replication of the Bacillus subtilis chromosome. IV. Genetic mapping by density transfer experiment. J Mol Biol. 1967 Jul 28;27(2):349–368. doi: 10.1016/0022-2836(67)90025-3. [DOI] [PubMed] [Google Scholar]
- Regan J. D., Setlow R. B., Ley R. D. Normal and defective repair of damaged DNA in human cells: a sensitive assay utilizing the photolysis of bromodeoxyuridine. Proc Natl Acad Sci U S A. 1971 Apr;68(4):708–712. doi: 10.1073/pnas.68.4.708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SACKS L. E., ALDERTON G. Behavior of bacterial spores in aqueous polymer two-phase systems. J Bacteriol. 1961 Sep;82:331–341. doi: 10.1128/jb.82.3.331-341.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAITO H., MIURA K. I. PREPARATION OF TRANSFORMING DEOXYRIBONUCLEIC ACID BY PHENOL TREATMENT. Biochim Biophys Acta. 1963 Aug 20;72:619–629. [PubMed] [Google Scholar]
- SETLOW R., BOYCE R. The action spectra for ultraviolet-light inactivation of systems-containing 5-bromouracil-substituted deoxyribonucleic acid. II. Bacteriophage T4. Biochim Biophys Acta. 1963 Mar 26;68:455–461. doi: 10.1016/0006-3002(63)90167-7. [DOI] [PubMed] [Google Scholar]
- Sakakibara Y., Ikeda Y. The dormant spore-specific state of DNA and macromolecular synthesis in spheroplasts of Bacillus subtilis spores. Biochim Biophys Acta. 1969 Apr 22;179(2):429–438. doi: 10.1016/0005-2787(69)90051-3. [DOI] [PubMed] [Google Scholar]
- Tanooka H., Sakakibara Y. Radioresistant nature of the transforming activity of DNA in bacterial spores. Biochim Biophys Acta. 1968 Jan 29;155(1):130–142. doi: 10.1016/0005-2787(68)90343-2. [DOI] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of Bacillus subtilis chromosome. I. Comparison of marker frequencies in exponential and stationary growth phases. Proc Natl Acad Sci U S A. 1963 Apr;49:559–566. doi: 10.1073/pnas.49.4.559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YOSHIKAWA H., SUEOKA N. Sequential replication of the Bacillus subtilis chromosome. II. Isotopic transfer experiments. Proc Natl Acad Sci U S A. 1963 Jun;49:806–813. doi: 10.1073/pnas.49.6.806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa H. The initiation of DNA replication in Bacillus subtilis. Proc Natl Acad Sci U S A. 1967 Jul;58(1):312–319. doi: 10.1073/pnas.58.1.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young F. E., Smith C., Reilly B. E. Chromosomal location of genes regulating resistance to bacteriophage in Bacillus subtilis. J Bacteriol. 1969 Jun;98(3):1087–1097. doi: 10.1128/jb.98.3.1087-1097.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]



