Abstract
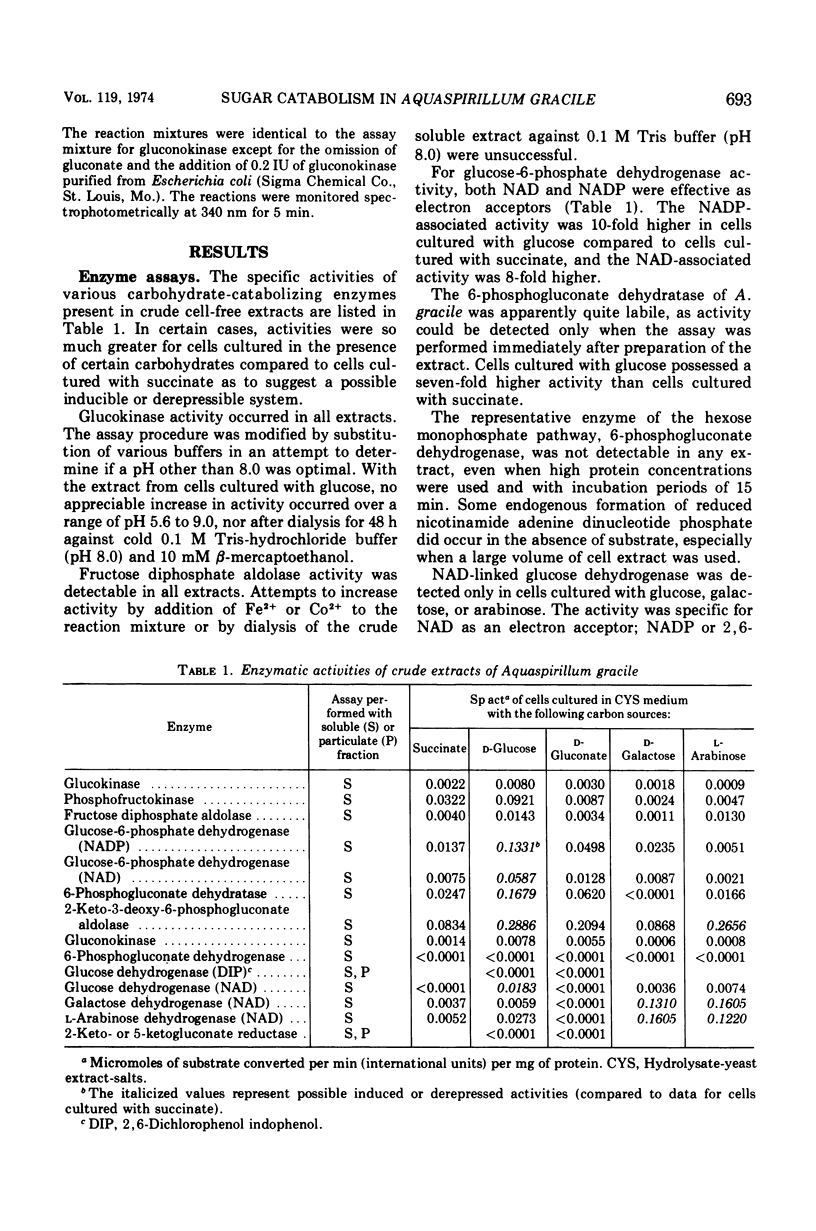
Aquaspirillum (Spirillum) gracile is one of the few spirilla that cause acidification of the medium when cultured with sugars. Acidic reactions have been reported only for d-glucose, d-galactose, and l-arabinose, and the mode of attack of these sugars has not been previously investigated. The soluble portion of extracts of glucose-cultured cells of A. gracile ATCC 19624 was found by spectrophotometric methods to contain enzyme activities characteristic of the Entner-Doudoroff and Embden-Meyerhof-Parnas pathways. No activity for 6-phosphogluconate dehydrogenase (EC 1.1.1.44) was detected. Pyridine nucleotide-linked dehydrogenase activities for l-arabinose and d-galactose (EC 1.1.1.46 and EC 1.1.1.48) occurred in the soluble fraction of cells cultured with either sugar. Glucose-cultured cells contained not only glucokinase (EC 2.7.1.2) and glucose-6-phosphate dehydrogenase (EC 1.1.1.49) activities but also glucose dehydrogenase (EC 1.1.1.47) activity. Enzymes capable of oxidizing gluconate were not detectable, but gluconokinase (EC 2.7.1.12) activity was present. Paper chromatographic analysis of the spent culture supernatant media from glucose-cultured cells indicated an accumulation of gluconic acid, and this was confirmed by enzymatic methods. Evidence is presented for the production of d-galactonic and l-arabonic acids in cultures containing d-galactose or l-arabinose, respectively.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Caraway B. H., Krieg N. R. Uncoordination and recoordination in Spirillum volutans. Can J Microbiol. 1972 Nov;18(11):1749–1759. doi: 10.1139/m72-271. [DOI] [PubMed] [Google Scholar]
- Cline A. L., Hu A. S. Some physical properties of three sugar dehydrogenases from a pseudomonad. J Biol Chem. 1965 Nov;240(11):4498–4502. [PubMed] [Google Scholar]
- DE LEY J., DEFLOOR J. 2-Ketogluconoreductase in micro-organisms. Biochim Biophys Acta. 1959 May;33(1):47–54. doi: 10.1016/0006-3002(59)90495-0. [DOI] [PubMed] [Google Scholar]
- Dahms A. S., Anderson R. L. 2-keto-3-deoxyl-L-arabonate aldolase and its role in a new pathway of L-arabinose degradation. Biochem Biophys Res Commun. 1969 Aug 22;36(5):809–814. doi: 10.1016/0006-291x(69)90681-0. [DOI] [PubMed] [Google Scholar]
- Dahms A. S., Anderson R. L. D-Fucose metabolism in a pseudomonad. II. Oxidation of D-fucose to D-fucono- -lactone by an L-arabino-aldose dehydrogenase and hydrolysis of the lactone by a lactonase. J Biol Chem. 1972 Apr 10;247(7):2228–2232. [PubMed] [Google Scholar]
- Dahms A. S., Anderson R. L. D-Fucose metabolism in a pseudomonad. IV. Cleavage of 2-keto-3-deoxy-D-fuconate to pyruvate and D-lactaldehyde by 2-keto-3-deoxy-L-arabonate aldolase. J Biol Chem. 1972 Apr 10;247(7):2238–2241. [PubMed] [Google Scholar]
- FISCHER F. G., DORFEL H. Die papierchromatographische Trennung und Bestimmung der Uronsäuren. Hoppe Seylers Z Physiol Chem. 1955 Sep 2;301(4-6):224–234. [PubMed] [Google Scholar]
- Hylemon P. B., Krieg N. R., Phibbs P. V., Jr Transport and catabolism of D-fructose by Spirillum itersomii. J Bacteriol. 1974 Jan;117(1):144–150. doi: 10.1128/jb.117.1.144-150.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hylemon P. B., Phibbs P. V., Jr Independent regulation of hexose catabolizing enzymes and glucose transport activity in Pseudomonas aeruginosa. Biochem Biophys Res Commun. 1972 Sep 5;48(5):1041–1048. doi: 10.1016/0006-291x(72)90813-3. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- WEIMBERG R., DOUDOROFF M. The oxidation of L-arabinose by Pseudomonas saccharophila. J Biol Chem. 1955 Dec;217(2):607–624. [PubMed] [Google Scholar]
- WEIMBERG R. Pentose oxidation by Pseudomonas fragi. J Biol Chem. 1961 Mar;236:629–635. [PubMed] [Google Scholar]
- WOOD W. A. Pathways of carbohydrate degradation in Pseudomonas fluorescens. Bacteriol Rev. 1955 Dec;19(4):222–233. doi: 10.1128/br.19.4.222-233.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]


