Abstract
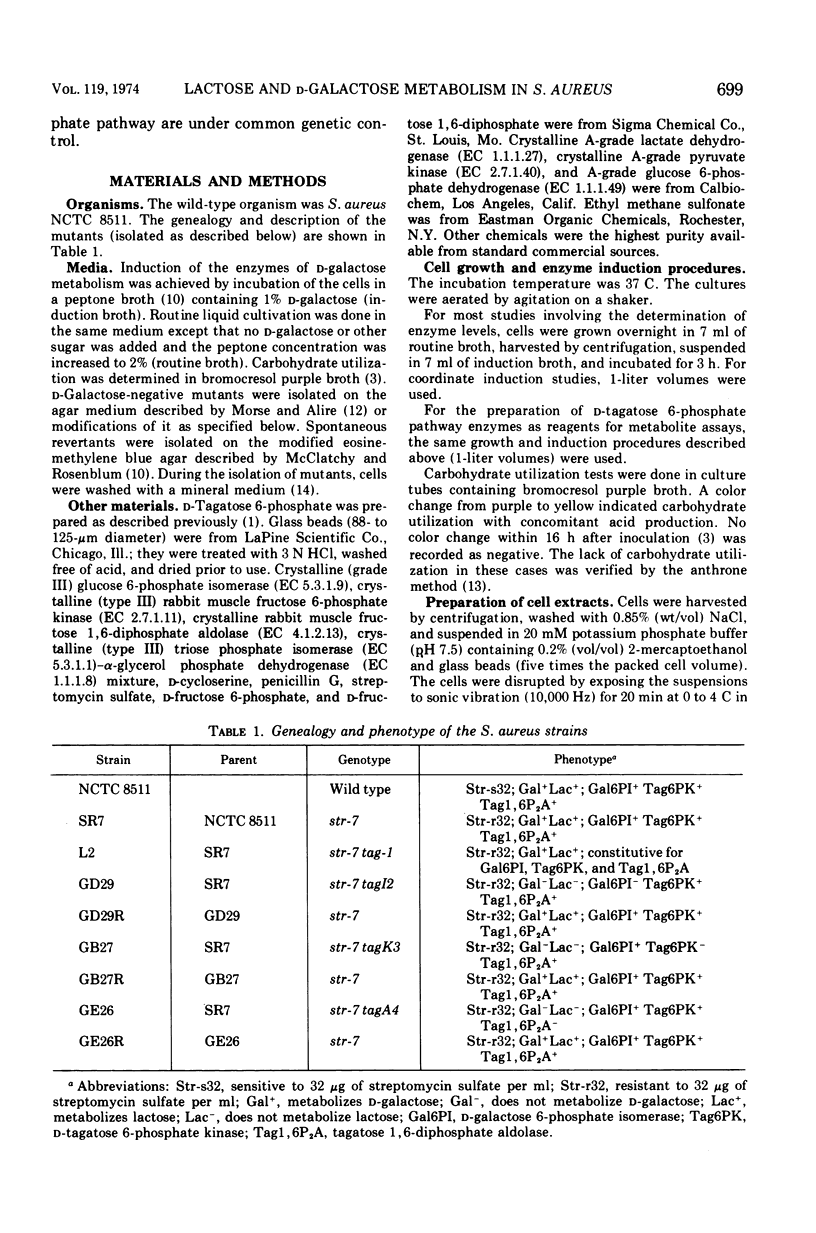
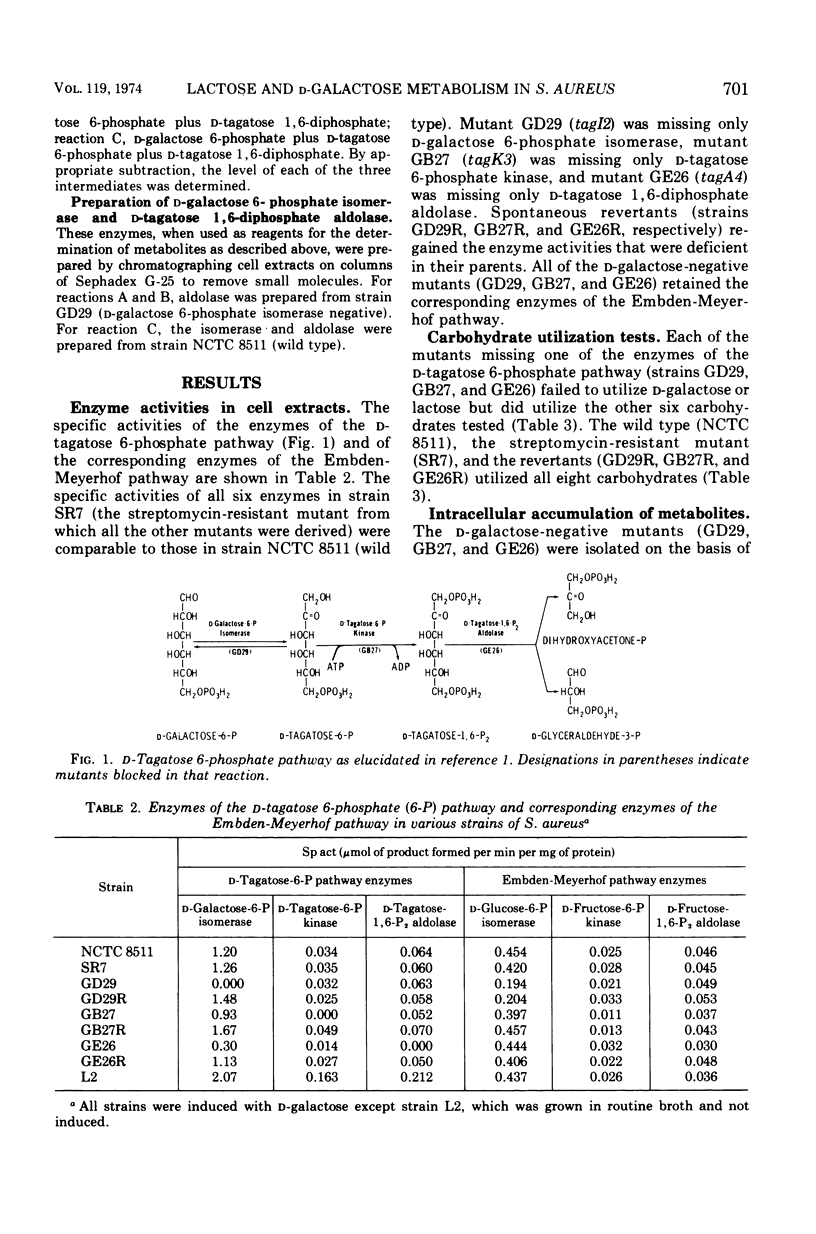
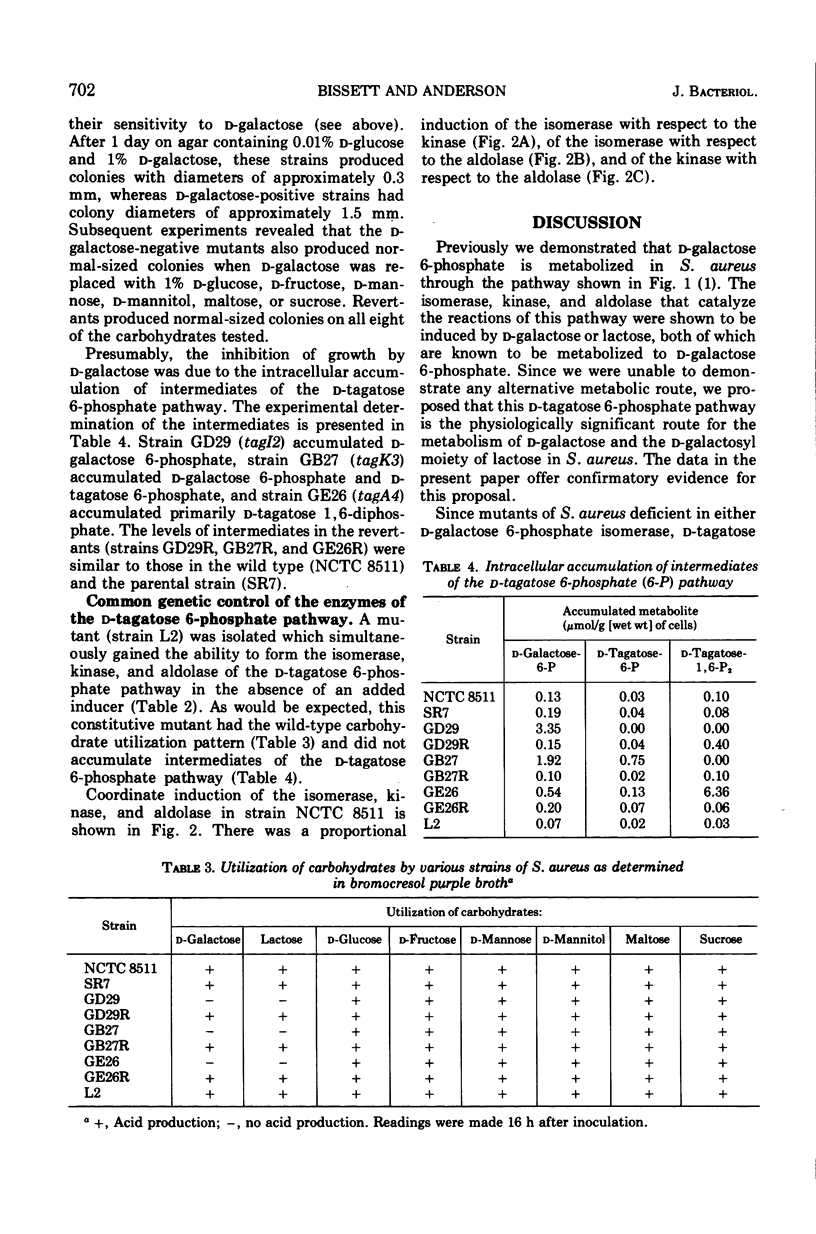
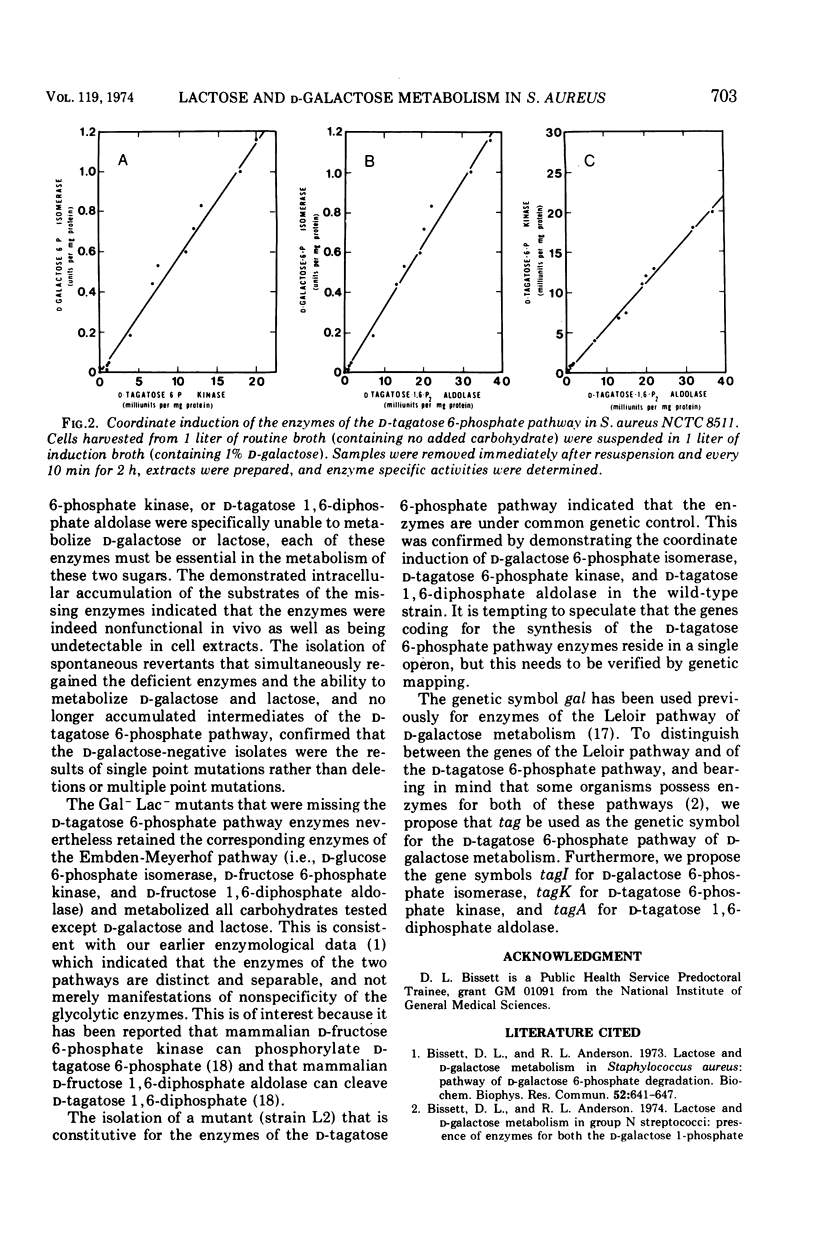
Mutants of Staphylococcus aureus were isolated which were unable to utilize d-galactose or lactose, but which were able to utilize all other carbohydrates tested. Growth of the mutants on a peptone-containing medium was inhibited by d-galactose. Of those mutants selected for further study, one (tagI2) was missing d-galactose 6-phosphate isomerase, one (tagK3) was missing d-tagatose 6-phosphate kinase, and one (tagA4) was missing d-tagatose 1, 6-diphosphate aldolase. Each of these mutants accumulated the substrate of the missing enzyme intracellularly. Spontaneous revertants of each of the mutants simultaneously regained their ability to utilize d-galactose and lactose, lost their sensitivity to d-galactose, regained the missing enzymatic activities, and no longer accumulated intermediates of the d-tagatose 6-phosphate pathway. These data support our previous contention that the physiologically significant route for the metabolism of d-galactose and the d-galactosyl moiety of lactose in S. aureus is the d-tagatose 6-phosphate pathway. Furthermore, a mutant constitutive for all three enzymes of this pathway was isolated, indicating that the products of the tagI, tagK, and tagA genes are under common genetic control. This conclusion was supported by the demonstration that d-galactose 6-phosphate isomerase, d-tagatose 6-phosphate kinase, and d-tagatose 1, 6-diphosphate aldolase are coordinately induced in the parental strain.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in group N streptococci: presence of enzymes for both the D-galactose 1-phosphate and D-tagatose 6-phosphate pathways. J Bacteriol. 1974 Jan;117(1):318–320. doi: 10.1128/jb.117.1.318-320.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D0galactose metabolism in Staphylococcus aureus: pathway of D-galactose 6-phosphate degradation. Biochem Biophys Res Commun. 1973 May 15;52(2):641–647. doi: 10.1016/0006-291x(73)90761-4. [DOI] [PubMed] [Google Scholar]
- EGAN J. B., MORSE M. L. CARBOHYDRATE TRANSPORT IN STAPHYLOCOCCUS AUREUS I. GENETIC AND BIOCHEMICAL ANALYSIS OF A PLEIOTROPIC TRANSPORT MUTANT. Biochim Biophys Acta. 1965 Feb 15;97:310–319. doi: 10.1016/0304-4165(65)90096-6. [DOI] [PubMed] [Google Scholar]
- Hengstenberg W., Egan J. B., Morse M. L. Carbohydrate transport in Staphylococcus aureus. V. The accumulation of phosphorylated carbohydrate derivatives, and evidence for a new enzyme-splitting lactose phosphate. Proc Natl Acad Sci U S A. 1967 Jul;58(1):274–279. doi: 10.1073/pnas.58.1.274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Morse M. L. Purification of the staphylococcal 6-phospho-beta-D-- galactosidase. Eur J Biochem. 1970 May 1;14(1):27–32. doi: 10.1111/j.1432-1033.1970.tb00256.x. [DOI] [PubMed] [Google Scholar]
- Kennedy E. P., Scarborough G. A. Mechanism of hydrolysis of O-nitrophenyl-beta-galactoside in Staphylococcus aureus and its significance for theories of sugar transport. Proc Natl Acad Sci U S A. 1967 Jul;58(1):225–228. doi: 10.1073/pnas.58.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOVELESS A., HOWARTH S. Mutation of bacteria at high levels of survival by ethyl methane sulphonate. Nature. 1959 Dec 5;184:1780–1782. doi: 10.1038/1841780a0. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MCCLATCHY J. K., ROSENBLUM E. D. INDUCTION OF LACTOSE UTILIZATION IN STAPHYLOCOCCUS AUREUS. J Bacteriol. 1963 Dec;86:1211–1215. doi: 10.1128/jb.86.6.1211-1215.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORSE M. L., ALIRE M. L. An agar medium indicating acid production. J Bacteriol. 1958 Sep;76(3):270–271. doi: 10.1128/jb.76.3.270-271.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sapico V., Hanson T. E., Walter R. W., Anderson R. L. Metabolism of D-fructose in Aerobacter aerogenes: analysis of mutants lacking D-fructose 6-phosphate kinase and D-fructose 1,6-diphosphatase. J Bacteriol. 1968 Jul;96(1):51–54. doi: 10.1128/jb.96.1.51-54.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S. Sugar transport. VII. Lactose transport in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):966–974. [PubMed] [Google Scholar]
- TUNG T. C., LING K. H., BYRNE W. L., LARDY H. A. Substrate specificity of muscle aldolase. Biochim Biophys Acta. 1954 Aug;14(4):488–494. doi: 10.1016/0006-3002(54)90228-0. [DOI] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]



