Abstract
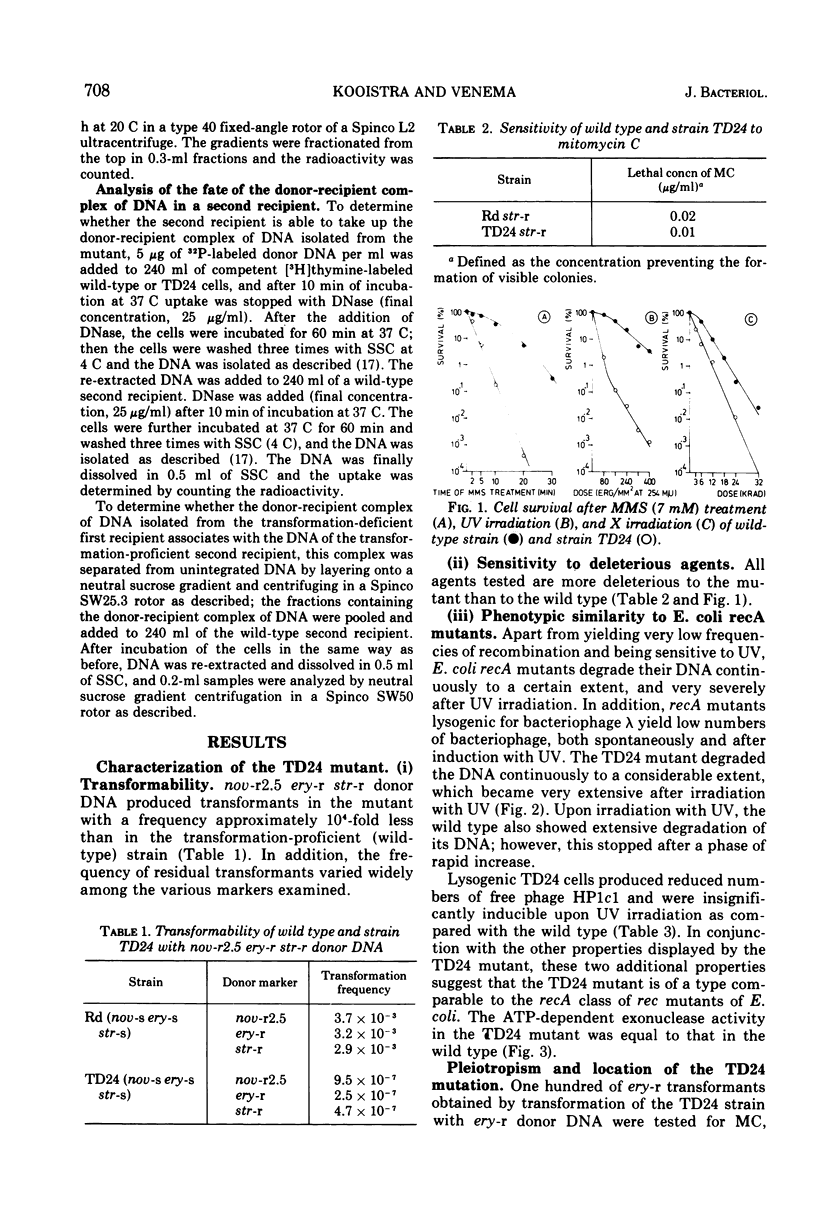
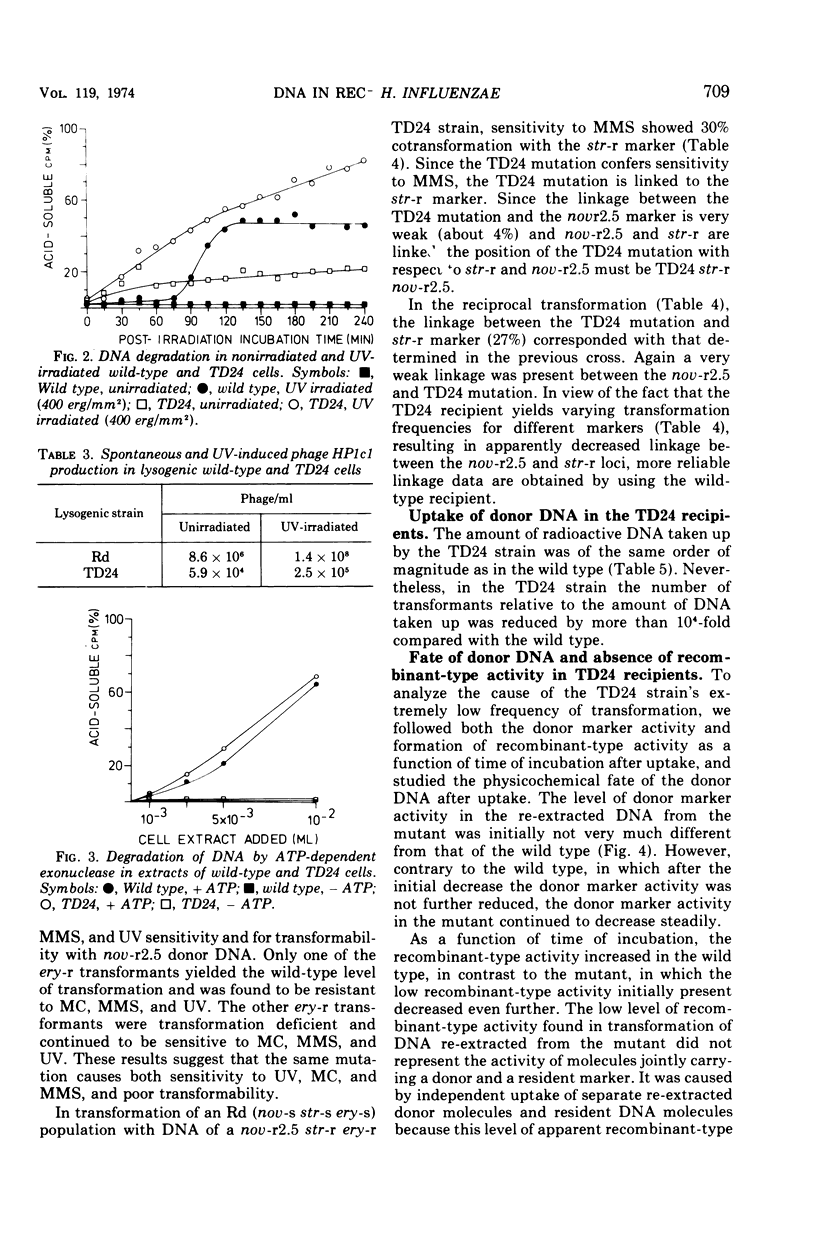
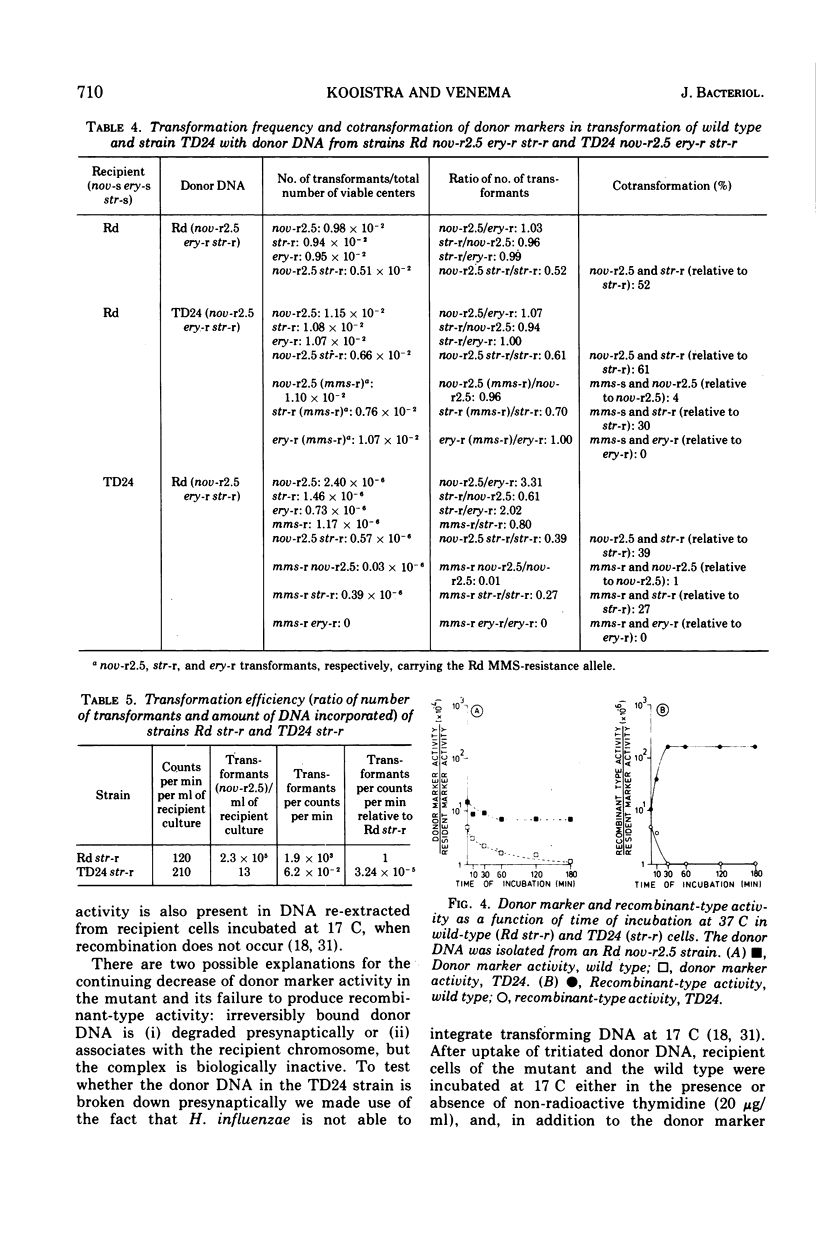
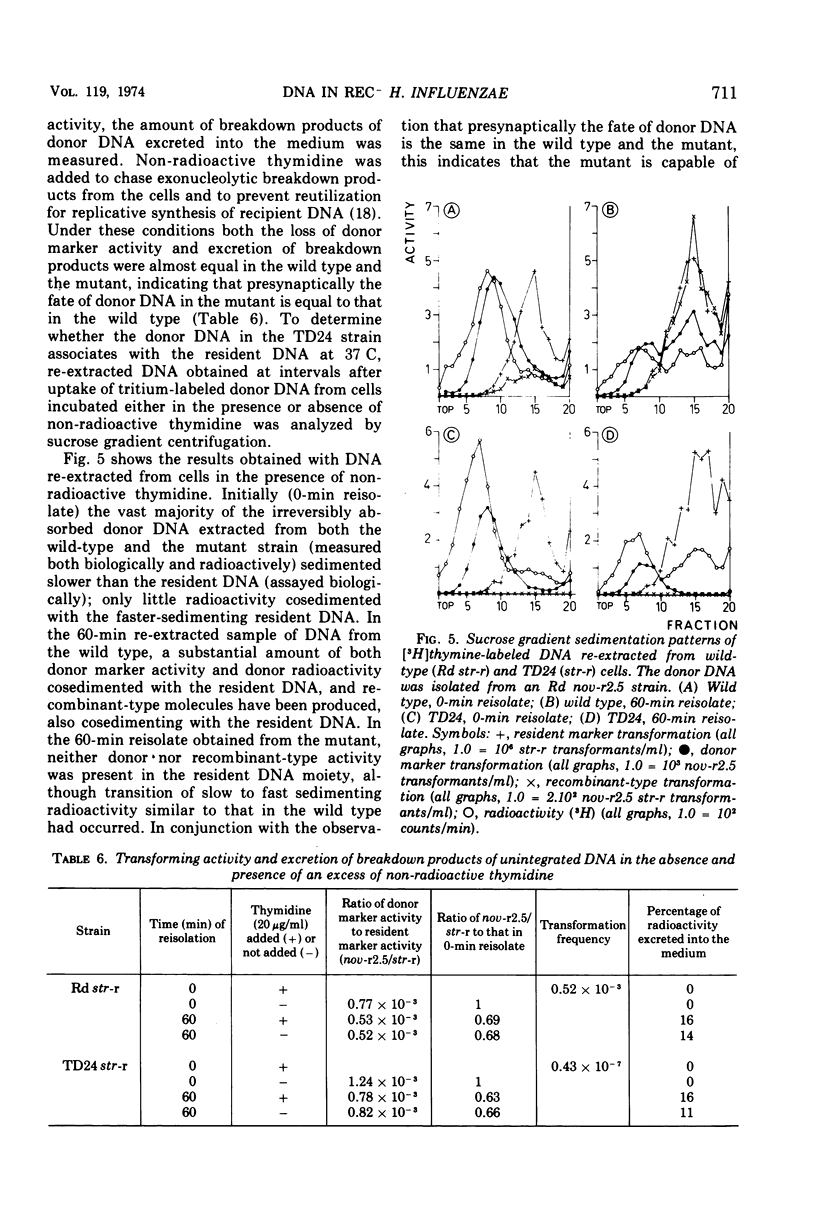
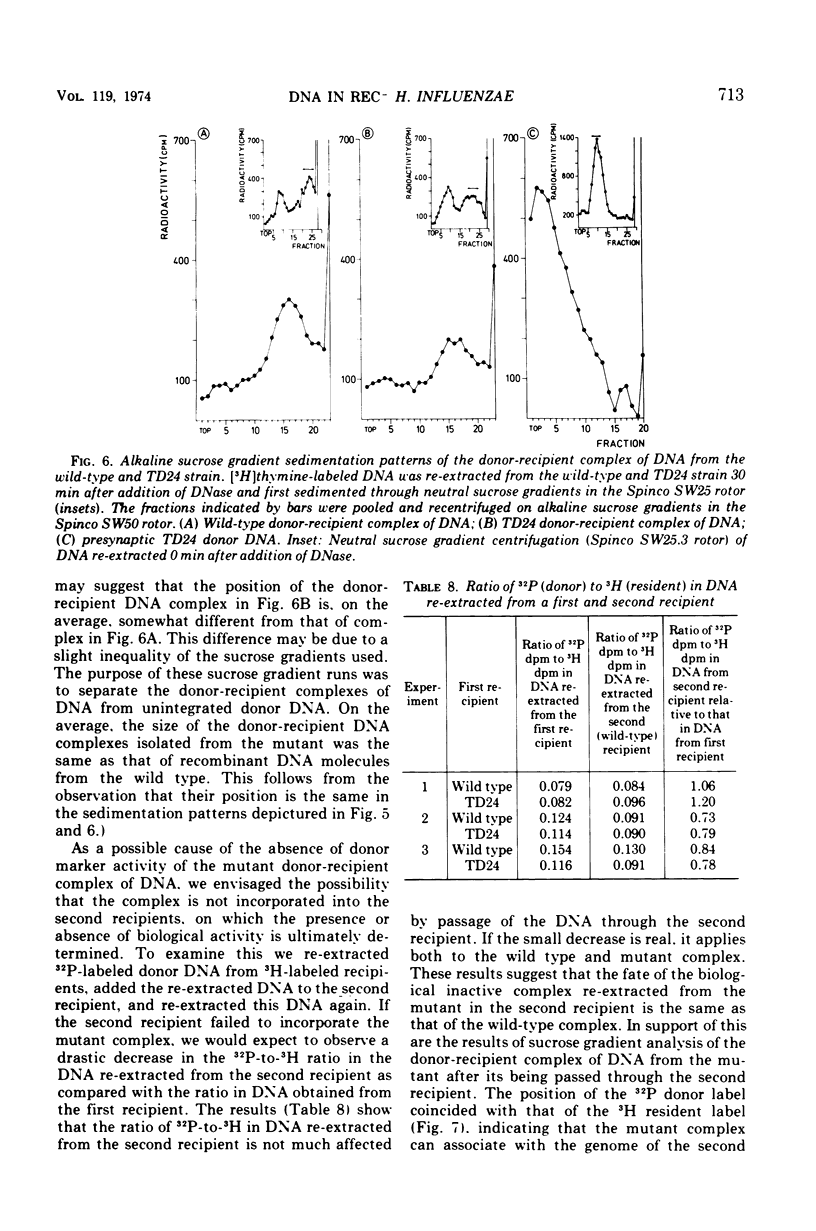
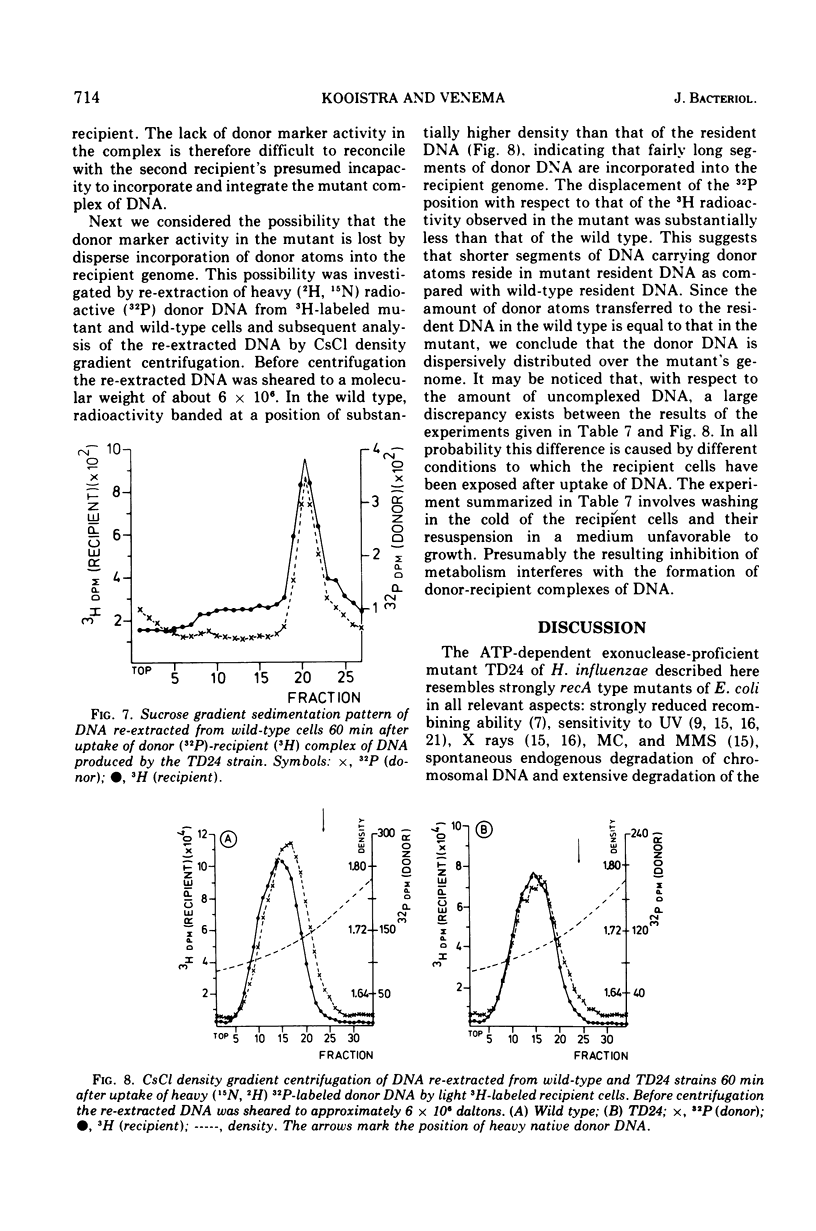
A transformation-deficient strain of Haemophilus influenzae (efficiency of transformation 104-fold less than that of the wild type), designated TD24, was isolated by selection for sensitivity to mitomycin C. In its properties the mutant was equivalent to recA type mutants of Escherichia coli. The TD24 mutation was linked with the str-r marker (about 30%) and only weakly linked with the nov-r2.5 marker. The uptake of donor deoxyribonucleic acid (DNA) was normal in the TD24 strain, but no molecules with recombinant-type activity (molecules carrying both the donor and the resident marker) were formed. In the mutant the intracellular presynaptic fate of the donor DNA was the same as that in the transformation-proficient (wild-type) strain, and the radioactive label of the donor DNA associated covalently with the recipient chromosome in about the same quantity as in the wild type. However, many fewer donor atoms were associated with segments of the mutant's recipient chromosome as compared with segments of the wild-type chromosome. In the mutant the association was accompanied by complete loss of donor marker activity. The lack of donor marker activity of the donor-recipient complex of DNA isolated from the mutant was not due to lack of uptake of the complex by the second recipient and its inability to associate with the second recipient's chromosome. Because the number of donor-atom-carrying resident molecules was higher than could be accounted for by the lengths of presynaptic donor molecules, we favor the idea that the association of donor DNA atoms with the mutant chromosome results from local DNA synthesis rather than from dispersive integration of donor DNA by recombination.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ALEXANDER H. E., LEIDY G. Determination of inherited traits of H. influenzae by desoxyribonucleic acid fractions isolated from type-specific cells. J Exp Med. 1951 Apr 1;93(4):345–359. doi: 10.1084/jem.93.4.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbour S. D., Clark A. J. Biochemical and genetic studies of recombination proficiency in Escherichia coli. I. Enzymatic activity associated with recB+ and recC+ genes. Proc Natl Acad Sci U S A. 1970 Apr;65(4):955–961. doi: 10.1073/pnas.65.4.955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks K., Clark A. J. Behavior of lambda bacteriophage in a recombination deficienct strain of Escherichia coli. J Virol. 1967 Apr;1(2):283–293. doi: 10.1128/jvi.1.2.283-293.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buttin G., Wright M. Enzymatic DNA degradation in E. coli: its relationship to synthetic processes at the chromosome level. Cold Spring Harb Symp Quant Biol. 1968;33:259–269. doi: 10.1101/sqb.1968.033.01.030. [DOI] [PubMed] [Google Scholar]
- CLARK A. J., MARGULIES A. D. ISOLATION AND CHARACTERIZATION OF RECOMBINATION-DEFICIENT MUTANTS OF ESCHERICHIA COLI K12. Proc Natl Acad Sci U S A. 1965 Feb;53:451–459. doi: 10.1073/pnas.53.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster J. H., Goodgal S. H. Competence Mutant of Haemophilus influenzae with Abnormal Ratios of Marker Efficiencies in Transformation. J Bacteriol. 1972 Oct;112(1):492–502. doi: 10.1128/jb.112.1.492-502.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caster J. H., Postel E. H., Goodgal S. H. Competence mutants: isolation of transformation deficient strains of Haemophilus influenzae. Nature. 1970 Aug 1;227(5257):515–517. doi: 10.1038/227515a0. [DOI] [PubMed] [Google Scholar]
- Clark A. J., Chamberlin M., Boyce R. P., Howard-Flanders P. Abnormal metabolic response to ultraviolet light of a recombination deficient mutant of Escherichia coli K12. J Mol Biol. 1966 Aug;19(2):442–454. doi: 10.1016/s0022-2836(66)80015-3. [DOI] [PubMed] [Google Scholar]
- Clark A. J. The beginning of a genetic analysis of recombination proficiency. J Cell Physiol. 1967 Oct;70(2 Suppl):165–180. doi: 10.1002/jcp.1040700412. [DOI] [PubMed] [Google Scholar]
- Davidoff-Abelson R., Dubnau D. Fate of transforming DNA after uptake by competent Bacillus subtilis: failure of donor DNA to replicate in a recombination-deficient recipient. Proc Natl Acad Sci U S A. 1971 May;68(5):1070–1074. doi: 10.1073/pnas.68.5.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Davidoff-Abelson R., Scher B., Cirigliano C. Fate of transforming deoxyribonucleic acid after uptake by competent Bacillus subtilis: phenotypic characterization of radiation-sensitive recombination-deficient mutants. J Bacteriol. 1973 Apr;114(1):273–286. doi: 10.1128/jb.114.1.273-286.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodgal S. H., Postel E. H. On the mechanism of integration following transformation with single-stranded DNA of Hemophilus influenzae. J Mol Biol. 1967 Sep 14;28(2):261–273. doi: 10.1016/s0022-2836(67)80008-1. [DOI] [PubMed] [Google Scholar]
- Hertman I., Luria S. E. Transduction studies on the role of a rec+ gene in the ultraviolet induction of prophage lambda. J Mol Biol. 1967 Jan 28;23(2):117–133. doi: 10.1016/s0022-2836(67)80021-4. [DOI] [PubMed] [Google Scholar]
- Hoch J. A., Barat M., Anagnostopoulos C. Transformation and transduction in recombination-defective mutants of Bacillus subtilis. J Bacteriol. 1967 Jun;93(6):1925–1937. doi: 10.1128/jb.93.6.1925-1937.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard-Flanders P., Boyce R. P. DNA repair and genetic recombination: studies on mutants of Escherichia coli defective in these processes. Radiat Res. 1966;(Suppl):156+–156+. [PubMed] [Google Scholar]
- Howard-Flanders P., Theriot L. Mutants of Escherichia coli K-12 defective in DNA repair and in genetic recombination. Genetics. 1966 Jun;53(6):1137–1150. doi: 10.1093/genetics/53.6.1137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kooistra J., Venema G. Fate of donor DNA in some poorly transformable strains of Haemophilus influenzae. Mutat Res. 1970 Mar;9(3):245–253. doi: 10.1016/0027-5107(70)90126-0. [DOI] [PubMed] [Google Scholar]
- Kooistra J., Venema G. Poor transformability with novr and eryr donor DNA of some mitomycin-C-sensitive strains of Haemophilus influenzae. Mutat Res. 1973 Dec;20(3):313–326. doi: 10.1016/0027-5107(73)90054-7. [DOI] [PubMed] [Google Scholar]
- Notani N. K., Setlow J. K., Joshi V. R., Allison D. P. Molecular basis for the transformation defects in mutants of Haemophilus influenzae. J Bacteriol. 1972 Jun;110(3):1171–1180. doi: 10.1128/jb.110.3.1171-1180.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa H., Shimada K., Tomizawa J. Studies on radiation-sensitive mutants of E. coli. I. Mutants defective in the repair synthesis. Mol Gen Genet. 1968 May 3;101(3):227–244. doi: 10.1007/BF00271625. [DOI] [PubMed] [Google Scholar]
- Oishi M. An ATP-dependent deoxyribonuclease from Escherichia coli with a possible role in genetic recombination. Proc Natl Acad Sci U S A. 1969 Dec;64(4):1292–1299. doi: 10.1073/pnas.64.4.1292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okubo S., Romig W. R. Impaired transformability of Bacillus subtilis mutant sensitive to mitomycin C and ultraviolet radiation. J Mol Biol. 1966 Feb;15(2):440–454. doi: 10.1016/s0022-2836(66)80120-1. [DOI] [PubMed] [Google Scholar]
- Postel E. H., Goodgal S. H. Competence mutants. 3. Responses to radiations. J Bacteriol. 1972 Jan;109(1):298–306. doi: 10.1128/jb.109.1.298-306.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postel E. H., Goodgal S. H. Competence mutants. II. Physical and biological fate of donor transforming deoxyribonucleic acid. J Bacteriol. 1972 Jan;109(1):292–297. doi: 10.1128/jb.109.1.292-297.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenthal P. N., Fox M. S. Effects of disintegration of incorporated 3H and 32P on the physical and biological properties of DNA. J Mol Biol. 1970 Dec 28;54(3):441–463. doi: 10.1016/0022-2836(70)90120-8. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Boling M. E., Beattie K. L., Kimball R. F. A complex of recombination and repair genes in Haemophilus influenzae. J Mol Biol. 1972 Jul 21;68(2):361–378. doi: 10.1016/0022-2836(72)90218-5. [DOI] [PubMed] [Google Scholar]
- Setlow J. K., Brown D. C., Boling M. E., Mattingly A., Gordon M. P. Repair of deoxyribonucleic acid in Haemophilus influenzae. I. X-ray sensitivity of ultraviolet-sensitive mutants and their behavior as hosts to ultraviolet-irradiated bacteriophage and transforming deoxyribonucleic acid. J Bacteriol. 1968 Feb;95(2):546–558. doi: 10.1128/jb.95.2.546-558.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuy J. H. Fate of transforming DNA in the Haemophilus influenzae transformation system. J Mol Biol. 1965 Sep;13(2):554–570. doi: 10.1016/s0022-2836(65)80117-6. [DOI] [PubMed] [Google Scholar]
- Stuy J. H. Phage resistance in Haemophilus influenzae. Biochem Biophys Res Commun. 1968 Nov 25;33(4):682–687. doi: 10.1016/0006-291x(68)90350-1. [DOI] [PubMed] [Google Scholar]
- Voll M. J., Goodgal S. H. Loss of activity of transforming deoxyribonucleic acid after uptake by Haemophilus influenzae. J Bacteriol. 1965 Oct;90(4):873–883. doi: 10.1128/jb.90.4.873-883.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vovis G. F. Adenosine triphosphate-dependent deoxyribonuclease from Diplococcus pneumoniae: fate of transforming deoxyribonucleic acid in a strain deficient in the enzymatic activity. J Bacteriol. 1973 Feb;113(2):718–723. doi: 10.1128/jb.113.2.718-723.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vovis G. F., Buttin G. An ATP-dependent deoxyribonuclease from Diplococcus pneumoniae. II. Evidence for its involvement in bacterial recombination. Biochim Biophys Acta. 1970 Nov 12;224(1):42–54. [PubMed] [Google Scholar]
- Willetts N. S., Clark A. J. Characteristics of some multiply recombination-deficient strains of Escherichia coli. J Bacteriol. 1969 Oct;100(1):231–239. doi: 10.1128/jb.100.1.231-239.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zadrazil S., Fucík V. Fate of transforming DNA in Bacillus subtilis strain sensitive to methyl methanesulfonate. Biochem Biophys Res Commun. 1971 Feb 19;42(4):676–683. doi: 10.1016/0006-291x(71)90541-9. [DOI] [PubMed] [Google Scholar]



