Abstract
In an attempt to improve behavioral memory, we devised a strategy to amplify the signal-to-noise ratio of the cAMP pathway, which plays a central role in hippocampal synaptic plasticity and behavioral memory. Multiple high-frequency trains of electrical stimulation induce long-lasting long-term potentiation, a form of synaptic strengthening in hippocampus that is greater in both magnitude and persistence than the short-lasting long-term potentiation generated by a single tetanic train. Studies using pharmacological inhibitors and genetic manipulations have shown that this difference in response depends on the activity of cAMP-dependent protein kinase A. Genetic studies have also indicated that protein kinase A and one of its target transcription factors, cAMP response element binding protein, are important in memory in vivo. These findings suggested that amplification of signals through the cAMP pathway might lower the threshold for generating long-lasting long-term potentiation and increase behavioral memory. We therefore examined the biochemical, physiological, and behavioral effects in mice of partial inhibition of a hippocampal cAMP phosphodiesterase. Concentrations of a type IV-specific phosphodiesterase inhibitor, rolipram, which had no significant effect on basal cAMP concentration, increased the cAMP response of hippocampal slices to stimulation with forskolin and induced persistent long-term potentiation in CA1 after a single tetanic train. In both young and aged mice, rolipram treatment before training increased long- but not short-term retention in freezing to context, a hippocampus-dependent memory task.
The second messenger, cAMP, and cAMP-dependent protein kinase A (PKA) have been implicated in short- and long-lasting synaptic plasticity in Aplysia and in short- and long-lasting behavioral learning in Aplysia and Drosophila (1, 2). Recently, convergent pharmacological and genetic evidence has also implicated the cAMP system in short-lasting long-term potentiation (LTP) at the mossy fiber–CA3 synapse of rodent hippocampus (3–6), and, strikingly, in the stronger longer-lasting intermediate and late phases of long-lasting LTP (L-LTP) that follow three to four trains of tetanic stimulation in all three hippocampal pathways: the perforant, the mossy fiber, and the Schaeffer collateral (CA3–CA1) (3, 7–14). LTP is a well studied example of synaptic plasticity in mammals, thought to be a candidate cellular mechanism for mediating some forms of explicit hippocampus-dependent memory (15, 16). L-LTP has been of particular interest in regard to this behavioral correlation, because it is much more persistent than the short-lasting long-term potentiation that follows a single tetanic train (7, 8, 9). L-LTP persists as long as it has been observed, up to 29 hr in vitro, and depends at later time points not only on PKA activity but also on transcription and translation (3, 6, 8, 9), much like behavioral long-term memory.
The dependence of L-LTP, in hippocampal slices and behavioral memory, on PKA activity suggests that increasing cAMP signaling might increase behavioral memory by raising the probability that long-lasting synaptic plasticity would occur after synaptic stimulation. However, administration of cAMP analogs such as Sp-cAMPS alone can cause long-lasting potentiation in rats that occludes subsequent electrical induction of L-LTP (11), suggesting that simply elevating cAMP throughout the hippocampus or brain might occlude rather than enhance synapse-specific strengthening. To avoid the possibility of such occlusion, we used low levels of phosphodiesterase (PDE) inhibition to maintain basal cAMP concentrations within the physiological range while selectively amplifying transient cAMP increases at active synapses.
Cyclic nucleotide PDEs are a large family of enzymes composed of at least 14 transcription units, many with alternately spliced isoforms (17). The PDEs have been grouped into seven families based on their regulation and substrate specificity, two of which, type IV and type VII, have cAMP as their nearly exclusive substrate. PDE inhibitors potentially can increase signaling through the cAMP system by inhibiting cAMP breakdown. Nonspecific PDE inhibitors, such as caffeine, have long been known to improve some behavioral performance in experimental animals (18, 19), although this appears to be caused by their antagonism of adenosine receptors or to their effects on intracellular Ca2+ stores. More recently, high doses of both nonspecific PDE inhibitors (papaverine, isobutylmethylxanthine) and type IV PDE-specific inhibitors (rolipram and Ro20–1724) have been found to improve memory in passive avoidance tasks in rodents when administered immediately, but not hours, after training (20, 21). These effects were postulated to be caused by increases in cAMP concentration in the brain, but neither their synaptic nor their biochemical basis was investigated.
MATERIALS AND METHODS
Biochemistry.
In each experiment, both hippocampi from a single C57\Bl6 mouse, male or female of 8–12 weeks of age, were dissected rapidly in iced oxygenated artificial cerebrospinal fluid (ACSF) consisting of (in mM): 124 NaCl/4.4 KCl/2.0 CaCl2/2.0 MgSO4/25 NaHCO3/1.0 Na2HPO4/10 glucose, sliced into 350-μm transverse sections on a tissue chopper, gradually warmed to 36.5°C in ACSF bubbled with 95% O2 and 5% CO2 and allowed to rest, submerged, for at least 1 hr. Slices were then transferred to oxygenated ACSF containing the indicated rolipram concentrations [in ACSF plus 0.1% dimethyl sulfoxide (DMSO)] for 30 min. Duplicate samples then were incubated in 5 μM forskolin (at a final concentration of 0.11% DMSO) or in 0.11% DMSO for 15 min. Eight experiments were performed, and duplicate individual slices were assayed for each data point. cAMP concentrations were determined by RIA (DuPont/NEN) according to the manufacturer’s instructions. After a logarithmic transformation, the data were analyzed by ANOVA followed by a Neuman–Keul multiple comparisons test.
Physiology.
Hippocampi from 8- to 12-week-old male C57\Bl6 mice were dissected rapidly in iced oxygenated ACSF, sliced into 400-μm transverse sections on a tissue chopper and allowed to rest in an interface chamber for at least 1.5 hr at a flow of about 1 ml per min. A bipolar nickel stimulating electrode and a glass recording electrode were placed in the stratum radiatum of area CA1 to record field excitatory postsynaptic potential (fEPSP). An input–output curve was used to set the baseline fEPSP at 35–40% of maximal slope, and half an hour of baseline data was gathered to assure stability of the preparation. In different slices interleaved in one series, rolipram or vehicle was added (in final concentration of 0.1% DMSO) to perfusate slices after 30 min and was perfused for 1 hr. When LTP was generated, the stimulus, 100 Hz × 1 s, was given at the midpoint of the PDE inhibitor infusion or just before its addition. The experimenters were blind to the contents of the solutions. The data were analyzed by Student’s unpaired t test.
Behavior.
Rolipram is absorbed fully and rapidly after oral administration in several species, including rat and human, although there is wide variability in first-pass metabolism (22). Based on studies of rolipram pharmacokinetics after oral and i.v. administration in rat and other species (22), we chose s.c. administration to avoid first-pass metabolism and calculated that a dose of 0.1 μmol/kg rolipram would yield a concentration between 0.06 μM and 0.2 μM in brain 30 min after treatment, based on a half-life of 1–3 hr and the observation that cerebrospinal fluid concentration was twice that in serum.
Nociception.
One-half hour before testing, 12- to 16-week-old male C57/Bl6 mice were injected by a blinded investigator with 0.1 μmol/kg rolipram in 10% Cremophor (BASF Bioresearch, Cambridge, MA)/PBS or with vehicle alone. Each mouse was placed in a chamber with a floor of metal bars and subjected to 1 s shocks of gradually increasing amperage (0.05, 0.1, 0.2, 0.3, 0.4, 0.5, 0.6, and 0.7 mA) at 1 min intershock intervals. Mice were scored for their first visible response to the shock (flinch), their first extreme motor response (run/jump), and their first vocalized distress (scream).
Open Field.
Starting 20 min after injection with the indicated dose of rolipram or vehicle alone by a blinded investigator, 12-week-old male C57/Bl6 mice were observed for 1 hr in a standard open field using video tracking (San Diego Instruments, San Diego, CA) and scored for path length, rearings, nose pokes, and proportion of time and path length in the center vs. periphery. Mice were returned for a second hour block after 24 hr.
Visible Platform Water Maze.
Mice were handled for 2 min daily for 7 days before training. On day one of training, mice were injected 30 min before their first trial with either 0.1 μmol/kg rolipram or vehicle alone. On three trials each day, each mouse was placed in the maze and allowed to swim for 1 min to reach a submerged platform marked with a black flag. Once the mouse reached the platform (or was helped there if he did not reach it on his own), he was allowed to rest there for 30 s, and was then held for 30 s while the platform was moved to a new random location, and the mouse started the next trial from a new location in the pool. Failures to reach the platform were scored as 60 s.
Freezing to Context.
One-half hour before training, 12- to 16-week-old male or female C57\Bl6 mice were injected in their home cages by a blinded investigator with the indicated concentration of rolipram in 10% Cremophor/PBS or with 10% Cremophor/PBS alone. For training, individual mice were placed in the training cage, where they spent 2.5 min exploring the new environment. During the last 30 s of the training period, a tone was sounded, and at the end of the tone, a shock of 0.4 mA × 2 s was delivered to the mouse through the bars of the floor. The mouse was then allowed to remain in the cage for 30 s more while immediate freezing was scored. Individual animals were returned to the training cage for scoring after either 1 or 24 hr. Freezing was scored by a blinded observer over a 5-min period divided into 5-sec intervals. A 5-s block was not scored as “frozen” if the animal moved its head or any limb during the block. The data were analyzed by Student’s unpaired t test.
RESULTS
Effects on cAMP Metabolism in Vitro.
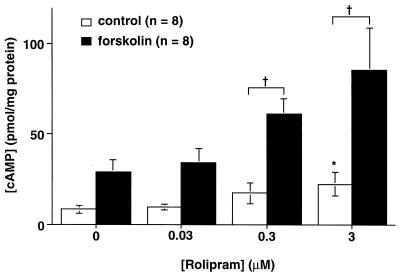
Because of the high level of expression of type IV PDE in the brain, and because the Drosophila type IV PDE homologue is the locus of the learning mutant dunce (23), we decided to use low concentrations of a type IV-specific PDE inhibitor, rolipram. We found that 45 min after the addition of low concentrations of this competitive inhibitor (0.03 or 0.3 μM), hippocampal slices showed no significant change in basal cAMP concentration (Fig. 1). Significant increases in basal cAMP concentration were observed only at a higher inhibitor concentration (3.0 μM). However, when adenylyl cyclase was stimulated by the addition of 5 μM forskolin to the bath, an effect of even low concentrations of rolipram was uncovered, and the increase in cAMP concentration in hippocampal slices in the presence of forskolin was significantly greater in the presence of 0.3 or 3 μM rolipram than in the presence of vehicle (43.5 ± 14.1 pmol cAMP per mg protein vs. 20.4 ± 8.7 for 0.3 μM rolipram-treated vs. vehicle-treated slices; P < 0.01).
Figure 1.
Effects of rolipram on cAMP metabolism of hippocampal slices. Low concentrations, 0.03 μM and 0.3 μM rolipram, had no significant effect on basal cAMP concentrations (open bars). However, the increase in cAMP concentration after 15 min of treatment with 5 μM forskolin (hatched bars) was significantly greater with 0.3 μM rolipram than with vehicle (†P < 0.01). At 3.0 μM rolipram, the forskolin-stimulated increases of cAMP were also significantly amplified (†P < 0.003 compared with control), but unstimulated basal cAMP concentrations were also significantly elevated (∗P < 0.001).
Effects on LTP.
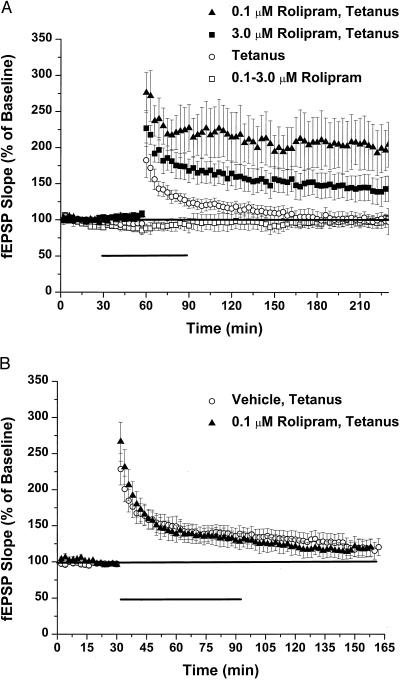
Rolipram (0.1 or 3.0 μM) had no effect on basal synaptic responses to stimulation of the Schaeffer collateral pathway in hippocampal slices (Fig. 2A). However, in the presence of 0.1 μM rolipram, a single train of tetanic stimulation induced increases in the slope of the field EPSP that were significantly larger than in control slices from early time points after the stimulus. fEPSP slopes then remained significantly elevated for at least 3 hr, a characteristic of the L-LTP that follows four tetanic trains in mouse (206 ± 38% vs. 99 ± 9% of baseline slope at 3 hr for 0.1 μM rolipram (n = 5) vs. vehicle (n = 5); P < 0.01). A higher dose of rolipram, 3.0 μM, also yielded LTP that was apparently stronger and longer lasting than controls after one train of stimulation, although not as effectively as 0.1 μM [139 ± 17% vs. 99 ± 9% of baseline slope at 3 hr for 3.0 μM rolipram (n = 6) vs. vehicle; not significant; 139 ± 17% vs. 206 ± 38% for 3.0 μM vs. 0.1 μM; P < 0.03].
Figure 2.
Effects of rolipram on synaptic transmission and LTP. Rolipram, 0.1 μM or 3.0 μM, had no significant effect on test fEPSPs generated at the CA3–CA1 synapse of C57\Bl6 hippocampal slices (A). However, when LTP was induced by a single tetanus of 100 Hz × 1 s at the same stimulus intensity in the presence of 0.1 μM or 0.3 μM rolipram, the resultant LTP was both larger and longer-lasting than in the absence of PDE inhibitor. Rolipram (0.1 μM) had no effect on the size or duration of LTP when perfused immediately after the stimulus (B).
Frey et al. (11) found that cAMP levels rose only transiently after generation of L-LTP, suggesting that the activation of cyclase was quite short lasting. Our experiments support the observation that cyclase activation is quite transient. To see any effect of rolipram on enhancing LTP, the drug had to be present at the time of tetanus; when rolipram was added immediately after tetanus, fEPSP potentiation returned to baseline with the same time course as in slices treated with a single train (n = 4; Fig. 2B). These results suggested that low concentrations of a specific PDE inhibitor potentiated and lengthened LTP by amplifying a cAMP transient that occurred after tetanic stimulation in slices, without affecting basal cAMP concentrations or synaptic transmission.
Behavioral Effects.
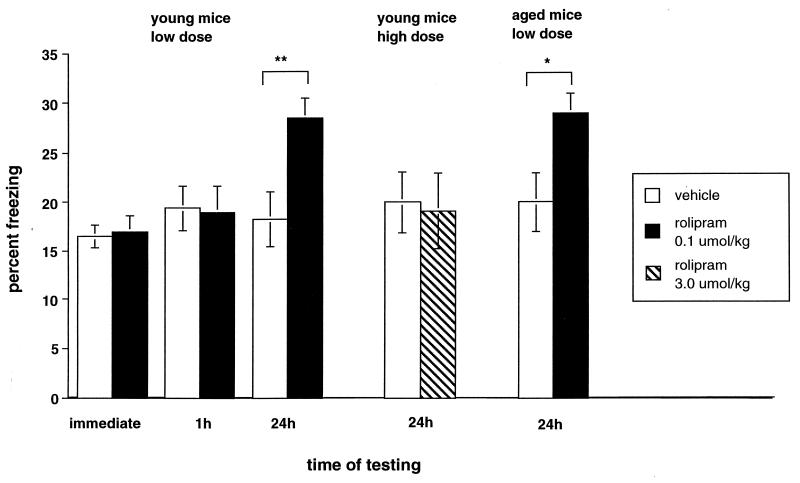
To test the effect of this manipulation on memory, we examined the effect of low-dose PDE inhibitor on context conditioning, a task that depends on the hippocampus (24, 25, 26). This task tests memory for a complex stimulus by quantifying an aspect of the fear response of an animal to an environment in which it previously received a weak electrical shock. We injected a dose of 0.1 μmol/kg rolipram calculated to produce an estimated concentration between 0.06 and 0.2 μM in brain 30 min after treatment and then trained the mice by exposing them to a novel environment for 2.5 min with a warning tone for the last 30 s before administering a mild footshock (0.4 mA for 2 s). We then measured the percentage of time mice spent freezing, defined as total immobility except for respiratory movements, immediately, 1 hr, or 24 hr after training (in the absence of footshock or other noxious stimulation, mice do not freeze). When tested immediately or 1 hr after training, there was no difference in freezing between rolipram- and vehicle-treated mice (Fig. 3; immediately: 17.0 ± 1.6% vs. 16.5 ± 1.2% for rolipram (n = 21) vs. controls (n = 23); not significant; 1 h: 19.0 ± 2.7% vs. 19.4 ± 2.3% for rolipram vs. controls (n = 12 each); not significant), suggesting that rolipram had no effect on aspects of performance such as perception of the environment, nociception, motor activity, or short-term memory. However, when tested for long-term memory 24 hr after training, rolipram-injected mice froze 62% more than vehicle-injected mice (28.5 ± 2.0% vs. 18.3 ± 2.8% for rolipram (n = 9) vs. controls (n = 11); P < 0.01, Student’s unpaired t test). Consistent with our hypothesis and our biochemical and physiological results, when mice were injected 30 min before training with 3 μmol/kg rolipram (calculated to yield a brain concentration between 2 and 6 μM, in the range that raised basal cAMP concentration in slices), the animals were quite lethargic during training (as previously reported, ref. 27), so that immediate and 1-hr freezing could not be measured and 24-hr retention was not at all enhanced (Fig. 3; 19.1 ± 3.9% vs. 20.0 ± 3.1% for 3 μmol/kg rolipram (n = 8) vs. vehicle (n = 9); not significant).
Figure 3.
Effects of rolipram on memory. Pretreatment with 0.1 μmol/kg rolipram had no effect on immediate freezing in the training chamber or on freezing to context when mice were returned to the training chamber after 1 hr. However, when mice were tested 24 hr later, rolipram increased freezing significantly over vehicle treatment alone (**P < 0.01). Injection with 3.0 μmol/kg rolipram before treatment had no effect on freezing to context after 24 hr. Pretreatment with 0.1 μmol/kg rolipram increased freezing to context in 18-month-old mice 24 hr after training (*P < 0.05).
In studies of memory, drugs frequently are given immediately after the training task to isolate effects that the drug may produce on aspects of performance, such as perception of pain (or other unconditioned stimulus) or of the environment (or other conditioned stimulus) from those on memory consolidation (28). Our physiological effect on LTP requires the presence of a PDE inhibitor during synaptic stimulation, while published memory-enhancing effects of rolipram and Ro20–1724 were observed when the drugs were given immediately after training, although these experiments used about 100-fold higher doses than we did (20, 21). We were able to reproduce this effect when we injected high-dose rolipram (3 μmol/kg) immediately after training, resulting in a 38% increase in freezing after 24 hr (29.8 ± 2.4% vs. 21.6 ± 2.1% for 3 μmol/kg rolipram (n = 16) vs. vehicle (n = 20); P < 0.02). We also observed an increase of freezing when we injected 0.1 μmol/kg rolipram after training (vs. 26.9 ± 5.6% vs. 12.9 ± 3.2% for 0.1 μmol/kg rolipram vs. vehicle (n = 12 each), P < 0.05).
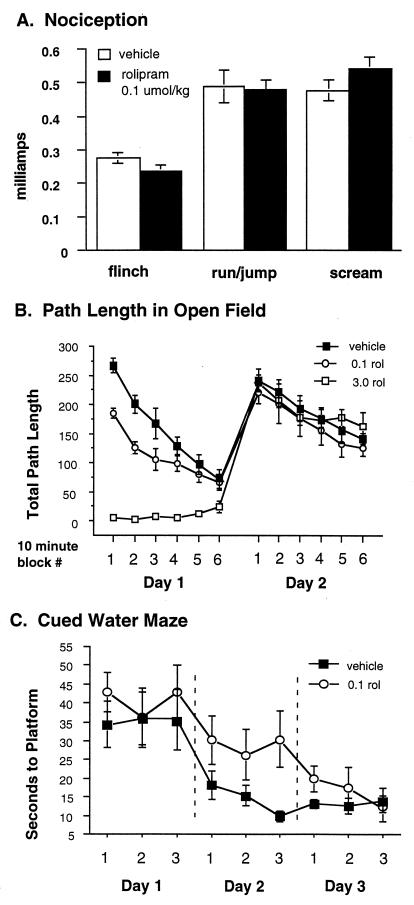
Rolipram might be increasing apparent memory by amplifying perception of the training shock or by decreasing the baseline activity of mice 24 hr after treatment. To control further for such nonspecific effects of rolipram, we examined the effects of rolipram on nociception and on open-field behavior as a measure of activity level. There was no significant effect of this low dose of rolipram on nociception 30 min after injection (Fig. 4A). In the open field, this dose of rolipram had no effect on motor activity at 70 or 80 min after the dose or on the next day, when the 1-hr and 24-hr tests of freezing were performed (Fig. 4B), although it decreased activity from 20 to 60 min after the dose. The effect of rolipram also appeared to be specific for hippocampus-dependent learning. Mice treated with rolipram (0.1 μmol/kg) 30 min before their first day of training in cued-platform water maze (29) showed slower learning and required an extra day to match the performance of vehicle-treated animals (Fig. 4C). Thus, performance on this task was, if anything, degraded rather than improved by rolipram. Despite these indications of marginal toxicity from this dose of rolipram, it actually led, in the contextual freezing task, to improved performance.
Figure 4.
Rolipram effects on hippocampus-independent behaviors. (A) Nociception: Injection of mice with 0.1 μmol/kg rolipram had no effect on nociception measured by average amperage to induce flinching, jumping, or running or crying by footshock. (B) Open field: Injection of mice with 0.1 μmol/kg or 3.0 μmol/kg rolipram affected open-field behavior only on the day of injection. Mice injected with 3.0 μmol/kg essentially lay motionless on the floor of the cage throughout the testing period on day one. Mice injected with 0.1 μmol/kg showed a modest decrease in activity from 20 to 60 min after injection but were identical to vehicle-injected controls by 70 min after injection. After 24 hr, there was no residual effect of either rolipram dose on open-field behavior. (C) Cued water maze: Injection of 0.1 μmol/kg 30 min before the first day of training on the visible platform water maze had no significant effect on first day behavior, but delayed acquisition of the task so that rolipram-treated animals required an extra day to match the behavior of vehicle-treated controls.
We also tested rolipram effects on memory in aged mice. Injection of 0.1 μmol/kg rolipram 30 min before training significantly increased freezing to context by aged animals after 24 hr (29 ± 2% vs. 20 ± 3% for vehicle injected (n = 6 each), P < 0.05, Fig. 3).
DISCUSSION
We have demonstrated that low doses of a type IV-specific PDE inhibitor can act to potentiate and extend LTP at the CA3–CA1 synapse in response to a stimulus that normally induces LTP lasting less than 1.5 hr. Consistent with earlier genetic experiments, our results support a correlation between L-LTP and long-term memory. Furthermore, the results suggest strongly that cAMP-driven increases in PKA activity are not only necessary for the transition from short-term to long-term processes at both the physiological and behavioral levels, as indicated by previous studies, but also can be sufficient to cause that transition, although we expect that other mechanisms may also be sufficient.
Whereas both low and high doses of rolipram facilitated the potentiation and temporal extension of LTP, low doses were more effective, suggesting that some occlusion was active at the higher concentration. Furthermore, only low doses were effective behaviorally in improving 24-hr memory when given before training. The failure of high doses to improve memory appears to be because high doses are behaviorally toxic, inducing lethargy and perhaps alterations in perception of the environment or some other process that prevents learning. Notably, behavioral improvements occur with a dose of rolipram at which cAMP signaling was increased although basal levels were left unchanged. Toxicity appeared at a dose that raised basal cAMP levels in our biochemical experiments. It is possible that toxicity in vivo may be independent of changes in basal cAMP concentrations through effects on targets other than type IV PDE, but rolipram appears to be particularly specific in this regard. Interestingly, effects of rolipram on the size of LTP starting immediately after training (Fig. 2A) were not reflected by increased freezing by animals at 1 hr. We are not sure what accounts for this observation. We speculate that the physiological LTP or LTP-like synaptic modification generated by the training may already saturate the contribution of synaptic strengthening to short-term memory. In vivo, it is clear also that the threshold for long-term memory has been reached in some pathways after this training with no rolipram present, because control animals also freeze at 24 hr, although for less time than rolipram-treated animals.
Several other agents have been described as type IV-specific agents. With one of these, Ro20–1724, we generated results in the in vitro biochemical and physiological experiments on cAMP signaling and L-LTP generation similar to those described above for rolipram (data not shown). However, injections of a wide range of doses of Ro20–1724 into living mice before training caused either no change in behavioral memory or lethargy suggestive of toxic effects, along with decreased 24-hr memory (data not shown). Consistent with these observations, Ro20–1724 has been reported to have toxic effects dissociated from its effects on cAMP, although rolipram has not been reported to have such effects (30, 31).
It is interesting that administration of both low and high doses of rolipram enhanced memory when administered after training, especially because the low dose had no effect on one-train LTP in vitro when perfused after the tetanus. Two explanations seem possible. One is that the mechanisms underlying LTP have nothing to do with the behavioral effect of rolipram treatment. However, it seems likely that behavioral training involves more than is modeled by a single tetanic train generating LTP in slice. Even when training consists of a single shock as unconditioned stimulus, animals are likely to replay or rehearse salient events in their experience during the process of memory consolidation. Because some 24-hr freezing occurs in the absence of rolipram, it is likely that the training experience in vivo more resembles the model of four tetanic trains spread over 15 min used to generate L-LTP in slice. Thus, even though given after training, rolipram might be present for one or more of the mental rehearsals of the experience. High-dose rolipram, by generally raising cAMP concentration throughout the brain, may enhance memory by a different mechanism when given after training, perhaps by consolidating changes at recently stimulated synapses “tagged” by endogenous signaling mechanisms (32, 33).
Our biochemical results indicate that it is possible to increase signaling in the cAMP pathway without significantly affecting basal cAMP concentrations. Targeting the degradative enzyme, in this case PDE, may be particularly fruitful because partial inhibition of degradation may be undetectable at basal levels of substrate, when the degradative enzyme is likely to be present in great excess, and homeostatic mechanisms may compensate for low levels of inhibition (34). Because rolipram is a competitive inhibitor with cAMP for PDE, Michaelis–Menton kinetics suggest that our results are most consistent with a model in which basal adenylyl cyclase activity is reduced to match that of the inhibited PDE [perhaps through phosphorylation by PKA (35, 36)], although retaining its normal potential for activation by forskolin. When signaling increases substrate concentration and stresses the capacity of the degradative enzyme, it uncovers the effect of the inhibitor and amplifies the size of the cAMP signal. Similar targeting of catabolic enzymes for low-level inhibition may be a fruitful means of amplifying other second-messenger signal to noise ratios for experimental and clinical purposes. Our experiments with chronic administration of rolipram to older animals (Mary Elizabeth Bach, M. B., Hyeon Sun, Min Zhuo, Yun-Fei Lu, Robert Shih, Isabelle Mansuy, Robert D. Hawkins and E.R.K., unpublished work) indicate that this treatment may be both efficacious and sufficiently free of toxic side effects to permit its clinical use to ameliorate age-related deficits in human memory.
Acknowledgments
We thank H. Wachtel, Kelsey Martin, and Ted Abel for sharing information and for helpful discussions, Daniela Brunner and Risa Fishman for assistance with statistical analysis, Alexander Glassman, George M. Martin, and Kelsey Martin for critical reading of the manuscript, and Harriet Ayers for typing the manuscript. This work was supported by a National Alliance for Research on Schizophrenia and Depression Young Investigator Award to M.B. and Established Investigator Award to E.R.K., and by the Howard Hughes Medical Institute.
ABBREVIATIONS
- fEPSP
field excitatory postsynaptic potential
- LTP
long-term potentiation
- L-LTP
long-lasting LTP
- PDE
phosphodiesterase
- PKA
cAMP-dependent protein kinase A
- ACSF
artificial cerebrospinal fluid
- DMSO
dimethyl sulfoxide
References
- 1.Goelet P, Castellucci V F, Schacher S, Kandel E R. Nature (London) 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- 2.Davis R L. Phys Rev. 1996;76:299–317. doi: 10.1152/physrev.1996.76.2.299. [DOI] [PubMed] [Google Scholar]
- 3.Huang Y Y, Nguyen P V, Abel T, Kandel E R. Learn Mem. 1996;3:74–85. doi: 10.1101/lm.3.2-3.74. [DOI] [PubMed] [Google Scholar]
- 4.Johnston D, Williams S, Jaffe D, Gray R. Annu Rev Physiol. 1992;54:489–505. doi: 10.1146/annurev.ph.54.030192.002421. [DOI] [PubMed] [Google Scholar]
- 5.Weiskopf M G, Casrillo P E, Zaluzky R A, Nicoll R A. Science. 1994;265:1878–1882. doi: 10.1126/science.7916482. [DOI] [PubMed] [Google Scholar]
- 6.Huang Y Y, Li X-C, Kandel E R. Cell. 1996;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 7.Frey U, Krug M, Reymann K G, Matthies H. Brain Res. 1988;452:57–65. doi: 10.1016/0006-8993(88)90008-x. [DOI] [PubMed] [Google Scholar]
- 8.Huang Y Y, Kandel E R. Learn Mem. 1994;1:74–82. [PubMed] [Google Scholar]
- 9.Nguyen P V, Abel T, Kandel E R. Science. 1994;265:1104–1107. doi: 10.1126/science.8066450. [DOI] [PubMed] [Google Scholar]
- 10.Abel T, Nguyen P V, Barad M, Deuel T A S, Kandel E R, Bourtchouladze R. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 11.Frey U, Huang Y Y, Kandel E R. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 12.Bourtchouladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva A J. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 13.Nguyen P V, Kandel E R. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Winder D G, Mansuy I M, Osman M, Moallem T M, Kandel E R. Cell. 1998;92:25–37. doi: 10.1016/s0092-8674(00)80896-x. [DOI] [PubMed] [Google Scholar]
- 15.Bliss T V, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 16.Butcher S P, Davis S, Morris R G. Eur Neuropsychopharmacol. 1990;1:15–20. doi: 10.1016/0924-977x(90)90005-u. [DOI] [PubMed] [Google Scholar]
- 17.Rybalkin S D, Beavo J A. Biochem Soc Trans. 1996;24:1005–1009. doi: 10.1042/bst0241005. [DOI] [PubMed] [Google Scholar]
- 18.Nicholson C D. Psychopharmacology. 1990;101:147–159. doi: 10.1007/BF02244119. [DOI] [PubMed] [Google Scholar]
- 19.Nehlig A, Daval J-L, Debry G. Brain Res Rev. 1992;17:139–170. doi: 10.1016/0165-0173(92)90012-b. [DOI] [PubMed] [Google Scholar]
- 20.Randt C T, Judge M E, Bonnet K A, Quartermain D. Pharmacol Biochem Behav. 1982;17:677–680. doi: 10.1016/0091-3057(82)90344-6. [DOI] [PubMed] [Google Scholar]
- 21.Villiger J W, Dunn A J. Behav Neural Biol. 1981;31:354–359. doi: 10.1016/s0163-1047(81)91424-2. [DOI] [PubMed] [Google Scholar]
- 22.Krause W, Kuhne G. Xenobiotica. 1988;18:561–571. doi: 10.3109/00498258809041693. [DOI] [PubMed] [Google Scholar]
- 23.Davis R L, Takayasu H, Eberwine M, Myres J. Proc Natl Acad Sci USA. 1989;86:3604–3608. doi: 10.1073/pnas.86.10.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Phillips R G, LeDoux J E. Behav Neurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- 25.Kim J J, Rison R A, Fanselow M S. Behav Neurosci. 1993;107:1093–1098. doi: 10.1037//0735-7044.107.6.1093. [DOI] [PubMed] [Google Scholar]
- 26.Logue S F, Paylor R, Wehner J M. Behav Neurosci. 1997;111:104–113. doi: 10.1037//0735-7044.111.1.104. [DOI] [PubMed] [Google Scholar]
- 27.Wachtel H. Psychopharmacology. 1982;77:309–316. doi: 10.1007/BF00432761. [DOI] [PubMed] [Google Scholar]
- 28.McGaugh J L. Brain Res Bull. 1989;23:339–345. doi: 10.1016/0361-9230(89)90220-7. [DOI] [PubMed] [Google Scholar]
- 29.Packard M G, White N M. Behav Neurosci. 1991;105:295–296. doi: 10.1037//0735-7044.105.2.295. [DOI] [PubMed] [Google Scholar]
- 30.Zurbonsen K, Michel A, Vittet D, Bonnet P-A, Chevillard C. Biochem Pharmacol. 1997;53:1141–1147. doi: 10.1016/s0006-2952(96)00822-2. [DOI] [PubMed] [Google Scholar]
- 31.Vicentini L M, Ambrosini A, Di Virgilio F, Meldolesi J, Pozzan T. Biochem J. 1986;234:555–562. doi: 10.1042/bj2340555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frey U, Morris R G M. Nature (London) 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 33.Martin K, Casadio A, Zhu H, E, Y, Rose J C, Chen M, Bailey C, Kandel E R. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- 34.Desauer C W, Posner B A, Gilman A G. Clin Sci. 1996;91:527–537. doi: 10.1042/cs0910527. [DOI] [PubMed] [Google Scholar]
- 35.Kunkel M W, Friedman J, Shenolikar S, Clark R B. FASEB J. 1989;3:2067–2074. doi: 10.1096/fasebj.3.9.2545497. [DOI] [PubMed] [Google Scholar]
- 36.Iwami G, Kawabe J, Ebina T, Cannon P J, Homey C J, Ishikawa Y. J Biol Chem. 1995;270:12481–12484. doi: 10.1074/jbc.270.21.12481. [DOI] [PubMed] [Google Scholar]