Abstract
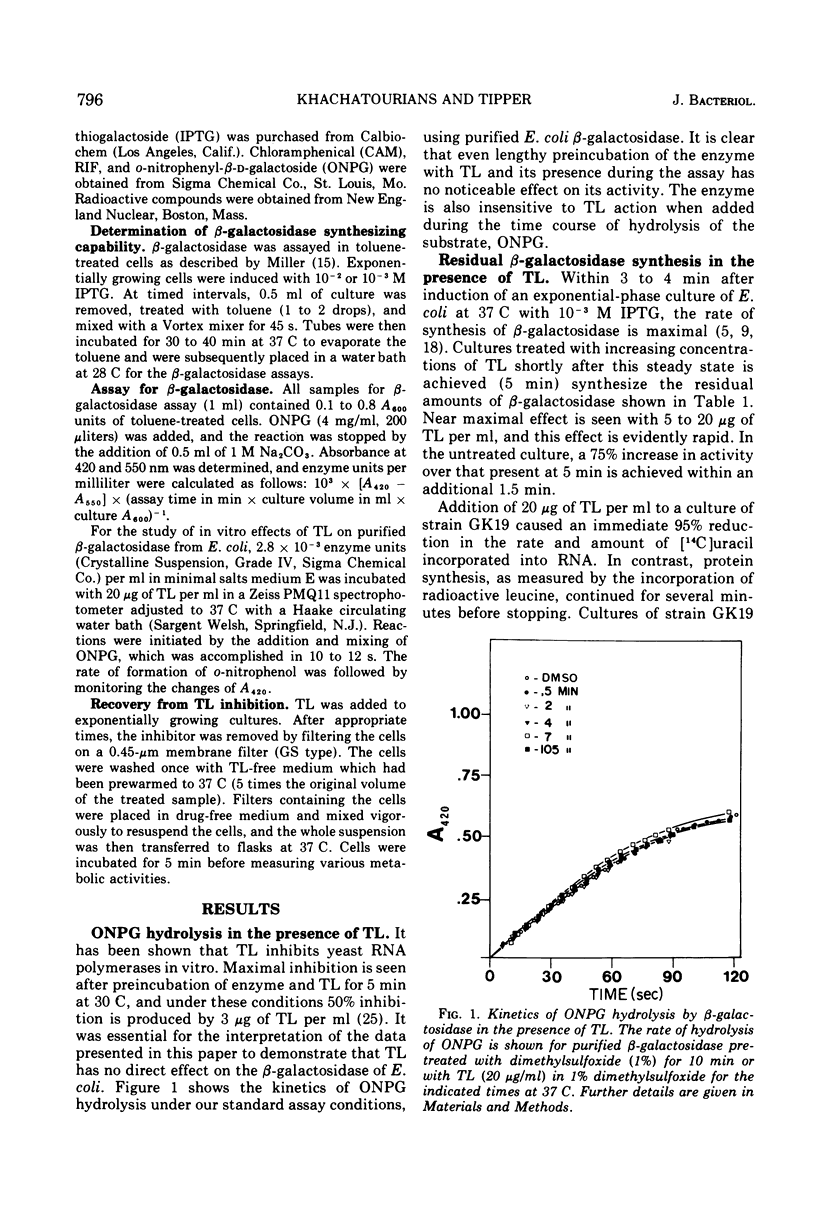
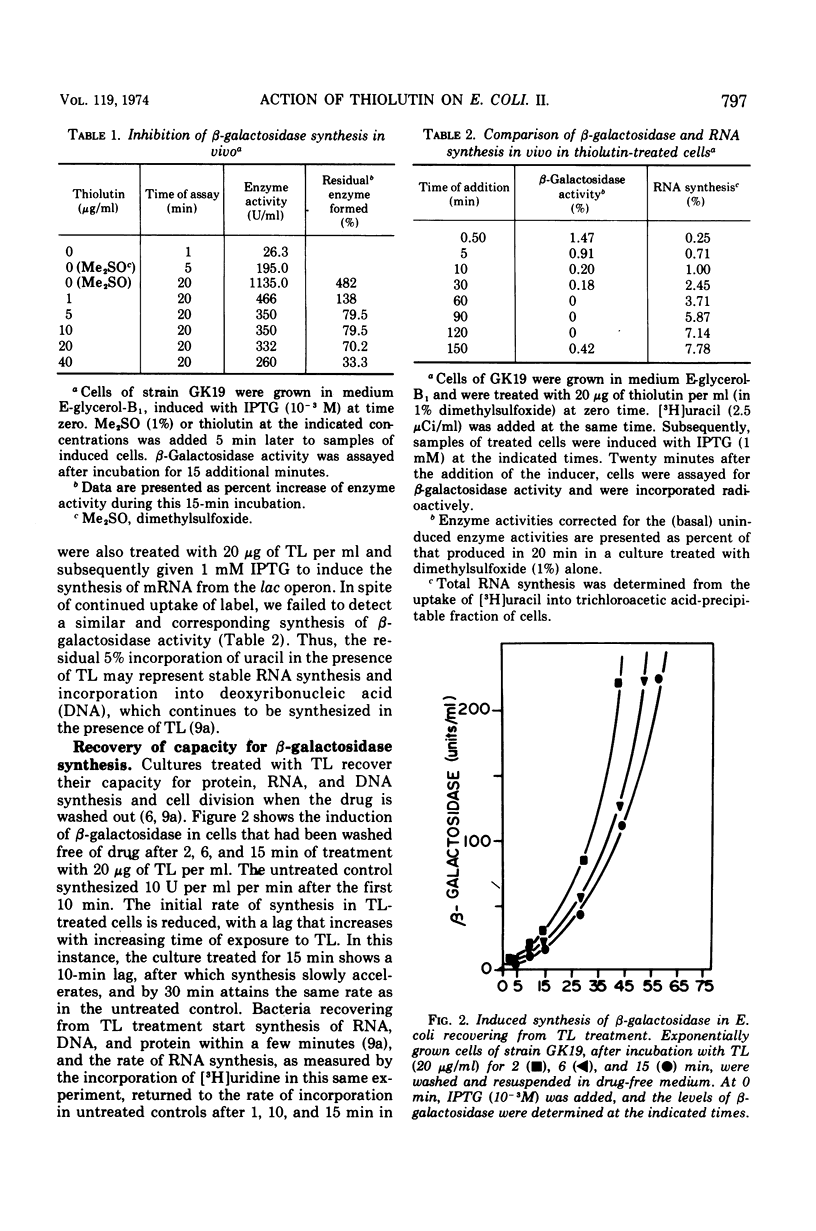
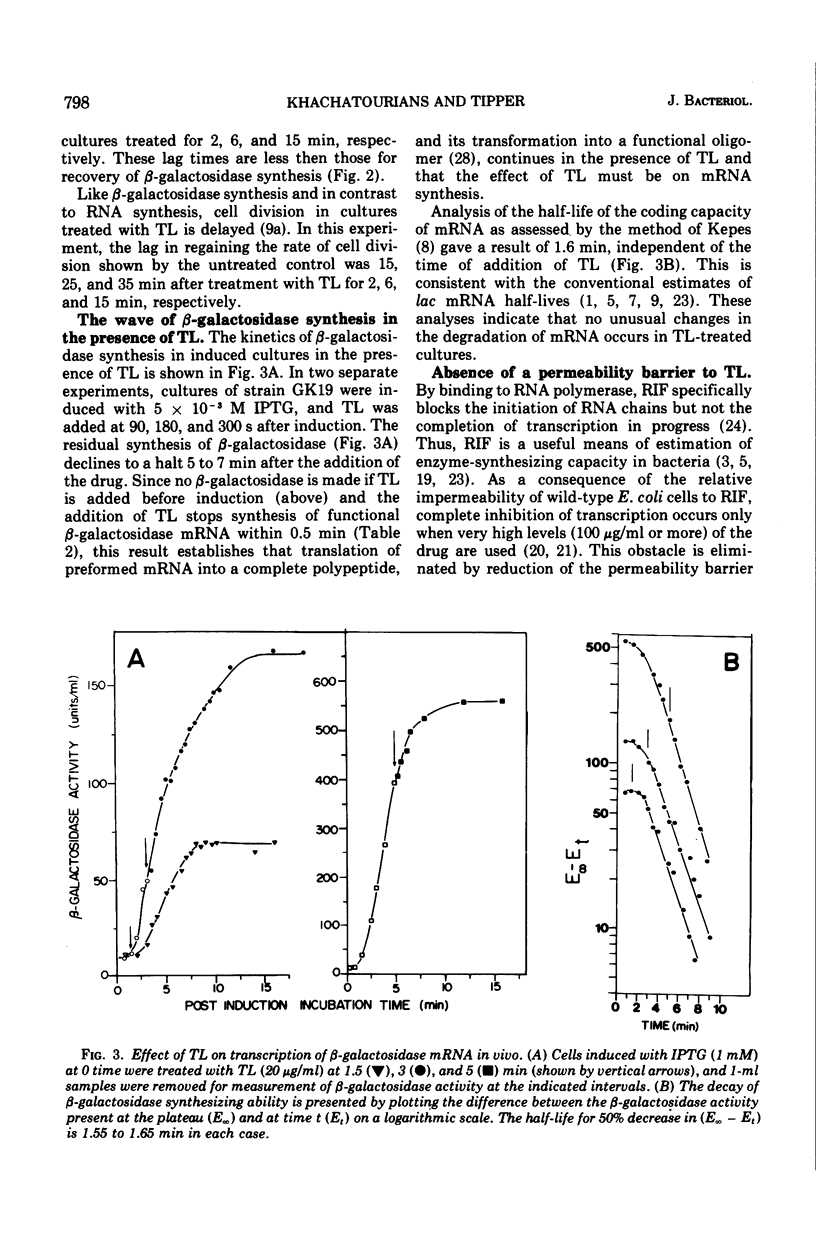
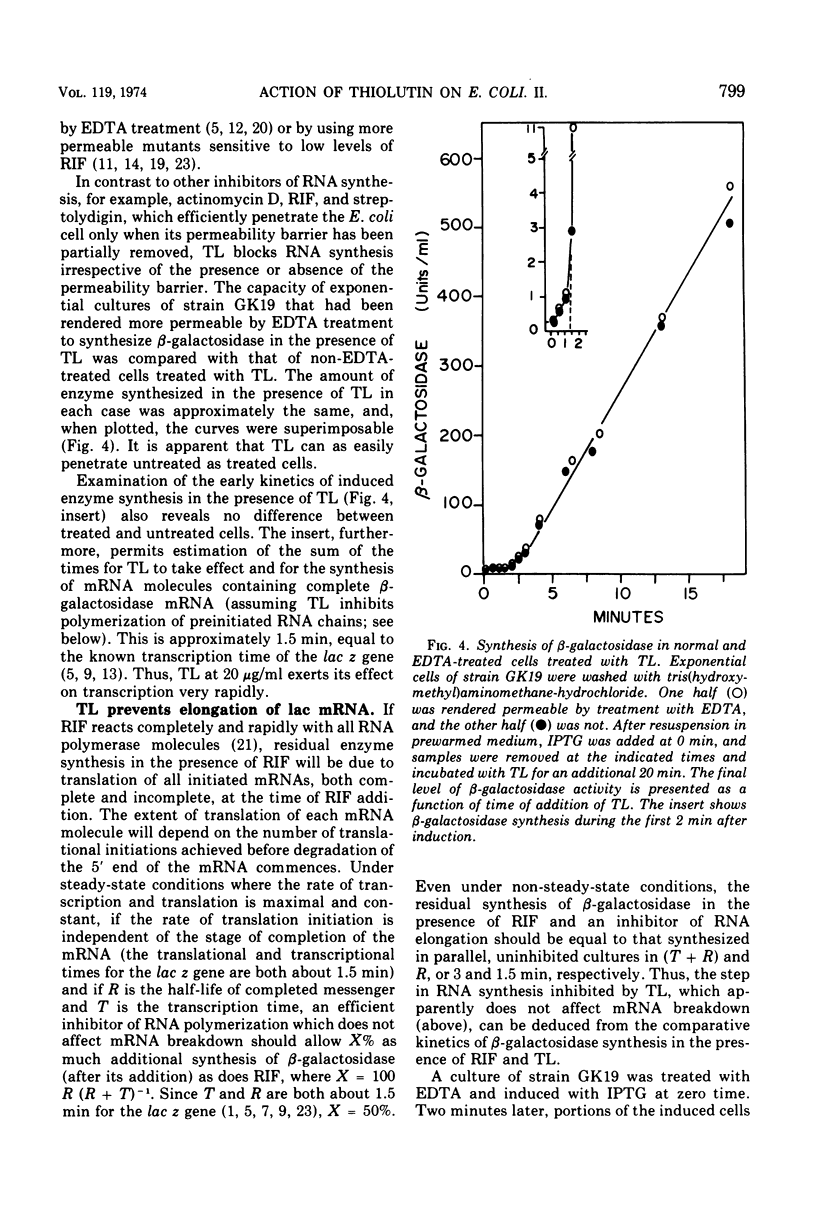
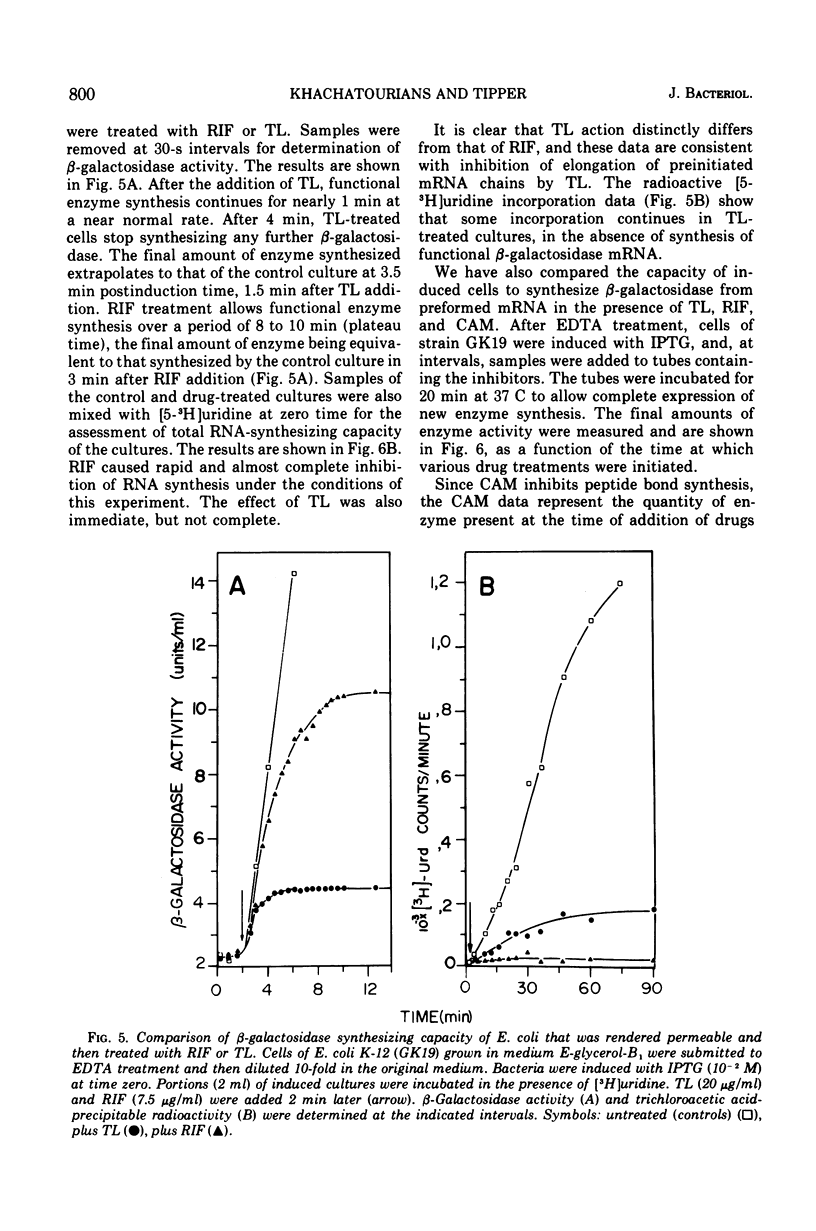
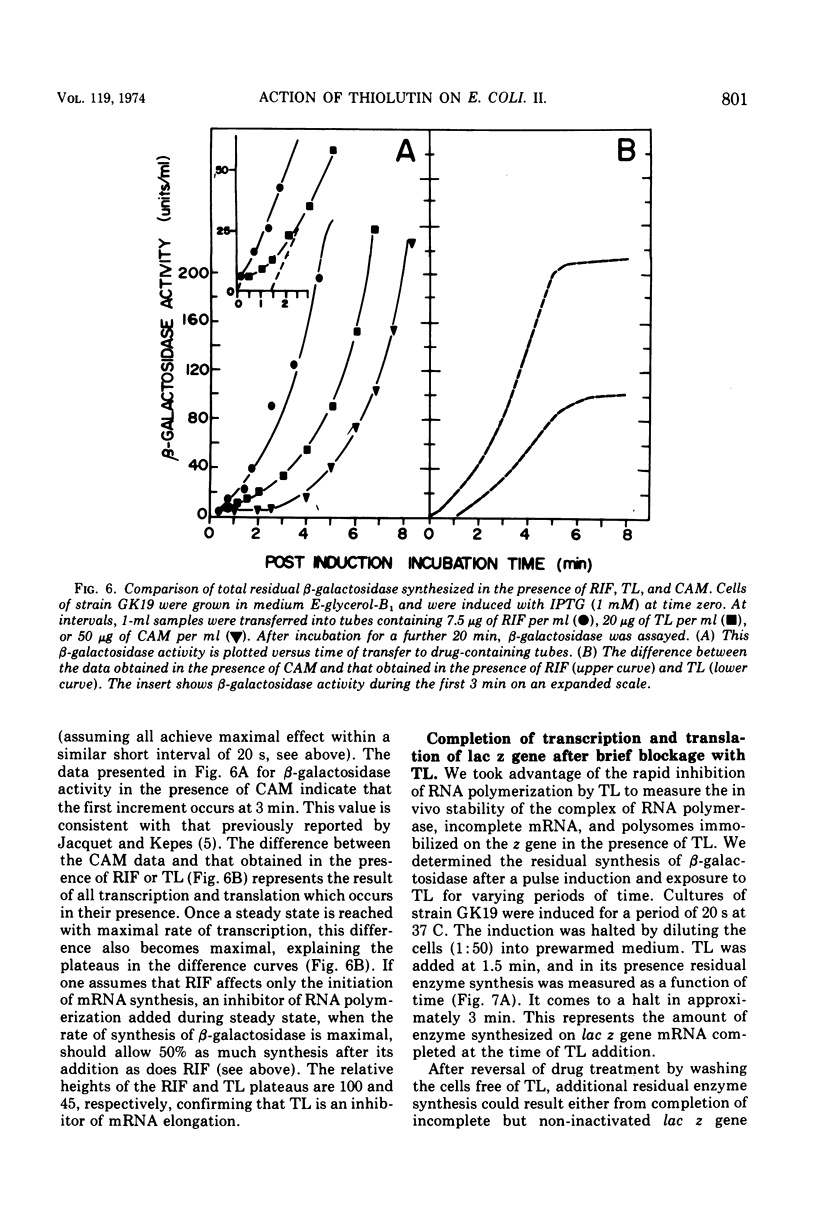
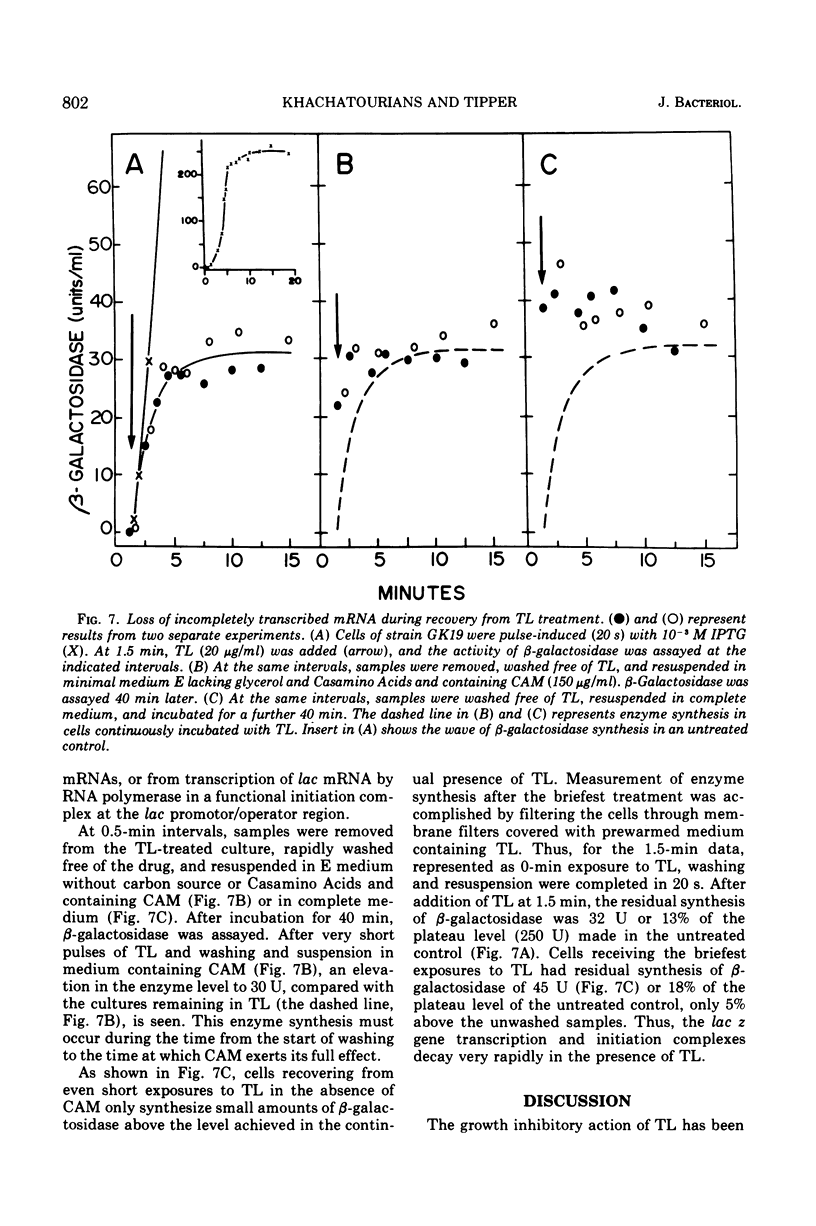
Thiolutin, at concentrations of 5 to 40 μg/ml, inhibited the induced synthesis of β-galactosidase in Escherichia coli CA8000. Thiolutin had no effect on the rate of in vitro hydrolysis of o-nitrophenyl-β-d-galactoside by purified β-galactosidase. Examination of the effects of thiolutin on the kinetics of appearance of β-galactosidase in the presence and absence of rifampin in induced E. coli cells indicated that thiolutin interferes with the transcription process at the level of elongation of messenger ribonucleic acid (mRNA) chains. The data indicated that, in the presence of thiolutin, β-galactosidase mRNA has a half-life of 1.6 min and that the first completed β-galactosidase mRNA is produced about 1.5 min after induction. These data are consistent with estimates of transcription time and messenger half-life obtained by conventional means, and suggest that thiolutin does not affect translation of mRNA or its breakdown in vivo. After removal of thiolutin, cells fully regained the ability to be induced for synthesis of β-galactosidase within 10 min, but mRNA which was incomplete at the time of thiolutin addition did not subsequently become functional.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Fan D. P. Decay of intact messengers in bacteria. J Mol Biol. 1966 Mar;16(1):164–179. doi: 10.1016/s0022-2836(66)80270-x. [DOI] [PubMed] [Google Scholar]
- Geiduschek E. P., Haselkorn R. Messenger RNA. Annu Rev Biochem. 1969;38:647–676. doi: 10.1146/annurev.bi.38.070169.003243. [DOI] [PubMed] [Google Scholar]
- Hall B. G., Gallant J. A. On the rate of messenger decay during amino acid starvation. J Mol Biol. 1973 Jan;73(1):121–124. doi: 10.1016/0022-2836(73)90163-0. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kepes A. Initiation, elongation and inactivation of lac messenger RNA in Escherichia coli studied studied by measurement of its beta-galactosidase synthesizing capacity in vivo. J Mol Biol. 1971 Sep 28;60(3):453–472. doi: 10.1016/0022-2836(71)90181-1. [DOI] [PubMed] [Google Scholar]
- Jacquet M., Kepes A. The step sensitive to catabolite repression and its reversal by 3'-5' cyclic AMP during induced synthesis of beta-galactosidase in E. coli. Biochem Biophys Res Commun. 1969 Jul 7;36(1):84–92. doi: 10.1016/0006-291x(69)90653-6. [DOI] [PubMed] [Google Scholar]
- Jimenez A., Tipper D. J., Davies J. Mode of action of thiolutin, an inhibitor of macromolecular synthesis in Saccharomyces cerevisiae. Antimicrob Agents Chemother. 1973 Jun;3(6):729–738. doi: 10.1128/aac.3.6.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KEPES A. KINETICS OF INDUCED ENZYME SYNTHESIS. DETERMINATION OF THE MEAN LIFE OF GALACTOSIDASE-SPECIFIC MESSENGER RNA. Biochim Biophys Acta. 1963 Oct 15;76:293–309. [PubMed] [Google Scholar]
- Kennell D., Bicknell I. Decay of messenger ribonucleic acid from the lactose operon of Escherichia coli as a function of growth temperature. J Mol Biol. 1973 Feb 15;74(1):21–31. doi: 10.1016/0022-2836(73)90351-3. [DOI] [PubMed] [Google Scholar]
- Kepes A. Transcription and translation in the lactose operon of Escherichia coli studied by in vivo kinetics. Prog Biophys Mol Biol. 1969;19(1):199–236. doi: 10.1016/0079-6107(69)90006-6. [DOI] [PubMed] [Google Scholar]
- Kuwano M., Kwan C. N., Apirion D., Schlessinger D. Ribonuclease V of escherichia coli. I. Dependence on ribosomes and translocation. Proc Natl Acad Sci U S A. 1969 Oct;64(2):693–700. doi: 10.1073/pnas.64.2.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. ACTINOMYCIN SENSITIVITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Biochem Biophys Res Commun. 1965 Jan 4;18:13–17. doi: 10.1016/0006-291x(65)90874-0. [DOI] [PubMed] [Google Scholar]
- Lark K. G. Evidence for the direct involvement of RNA in the initiation of DNA replication in Escherichia coli 15T. J Mol Biol. 1972 Feb 28;64(1):47–60. doi: 10.1016/0022-2836(72)90320-8. [DOI] [PubMed] [Google Scholar]
- Matzura H., Molin S., Maaloe O. Sequential biosynthesis of the and ' subunits of the DNA-dependent RNA polymerase from Escherichia coli. J Mol Biol. 1971 Jul 14;59(1):17–25. doi: 10.1016/0022-2836(71)90410-4. [DOI] [PubMed] [Google Scholar]
- Morikawa N., Imamoto F. Degradation of tryptophan messenger. On the degradation of messenger RNA for the tryptophan operon in Escherichia coli. Nature. 1969 Jul 5;223(5201):37–40. doi: 10.1038/223037a0. [DOI] [PubMed] [Google Scholar]
- Morse D. E., Mosteller R. D., Yanofsky C. Dynamics of synthesis, translation, and degradation of trp operon messenger RNA in E. coli. Cold Spring Harb Symp Quant Biol. 1969;34:725–740. doi: 10.1101/sqb.1969.034.01.082. [DOI] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. The initial kinetics of enzyme induction. Biochim Biophys Acta. 1961 Apr 29;49:77–88. doi: 10.1016/0006-3002(61)90871-x. [DOI] [PubMed] [Google Scholar]
- Pato M. L., Bennett P. M., von Meyenburg K. Messenger ribonucleic acid synthesis and degradation in Escherichia coli during inhibition of translation. J Bacteriol. 1973 Nov;116(2):710–718. doi: 10.1128/jb.116.2.710-718.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riva S., Silvestri L. G. Rifamycins: a general view. Annu Rev Microbiol. 1972;26:199–224. doi: 10.1146/annurev.mi.26.100172.001215. [DOI] [PubMed] [Google Scholar]
- Rogerson A. C., Ezekiel D. H. Decay of ribonucleic acid synthesis in amino acid-starved Escherichia coli after rifampin treatment. J Bacteriol. 1974 Mar;117(3):987–993. doi: 10.1128/jb.117.3.987-993.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scaife J. G. Control of transcription in bacteria. Br Med Bull. 1973 Sep;29(3):214–219. doi: 10.1093/oxfordjournals.bmb.a071010. [DOI] [PubMed] [Google Scholar]
- Schwartz T., Craig E., Kennell D. Inactivation and degradation of messenger ribnucleic acid from the lactose operon of Escherichia coli. J Mol Biol. 1970 Dec 14;54(2):299–311. doi: 10.1016/0022-2836(70)90431-6. [DOI] [PubMed] [Google Scholar]
- Tipper D. J. Inhibition of yeast ribonucleic acid polymerases by thiolutin. J Bacteriol. 1973 Oct;116(1):245–256. doi: 10.1128/jb.116.1.245-256.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]
- Varmus H. E., Perlman R. L., Pastan I. Regulation of lac transcription in antibiotic-treated E. coli. Nat New Biol. 1971 Mar 10;230(10):41–44. doi: 10.1038/newbio230041a0. [DOI] [PubMed] [Google Scholar]


