Abstract
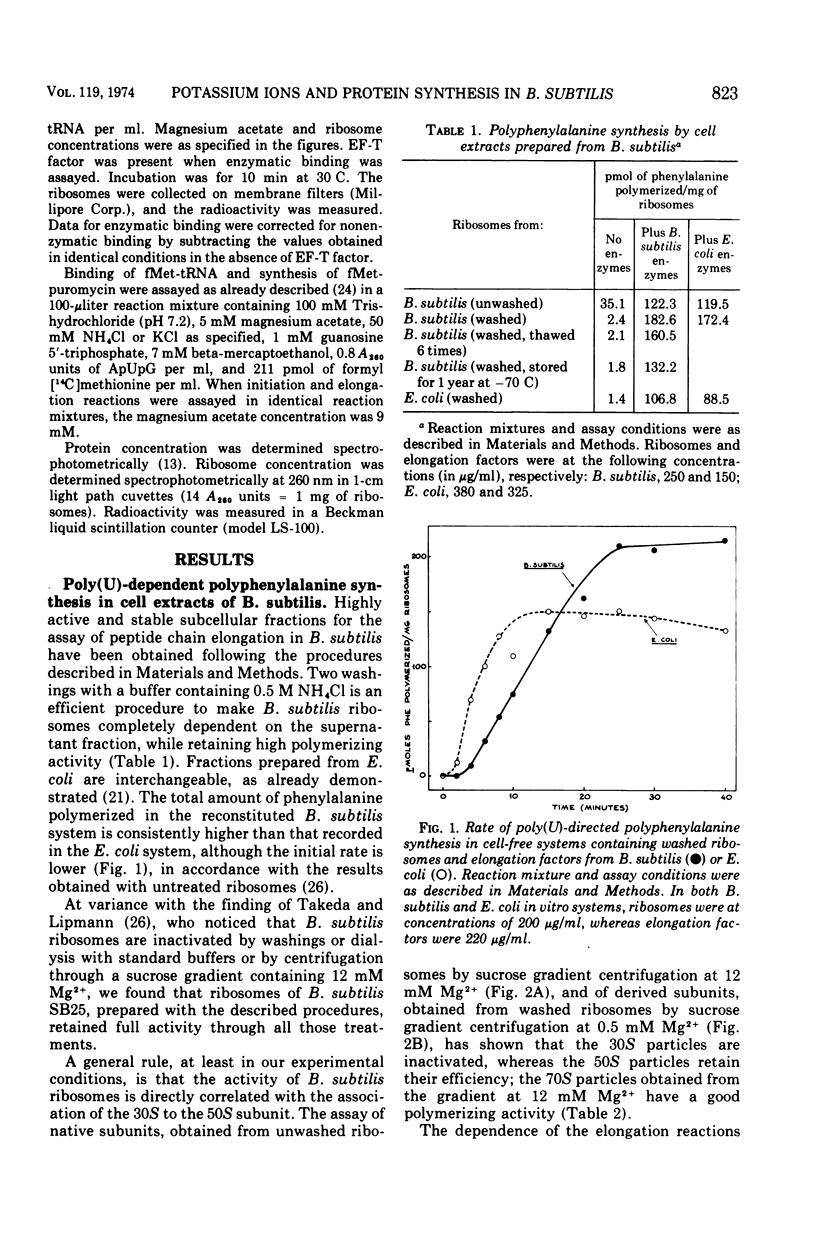
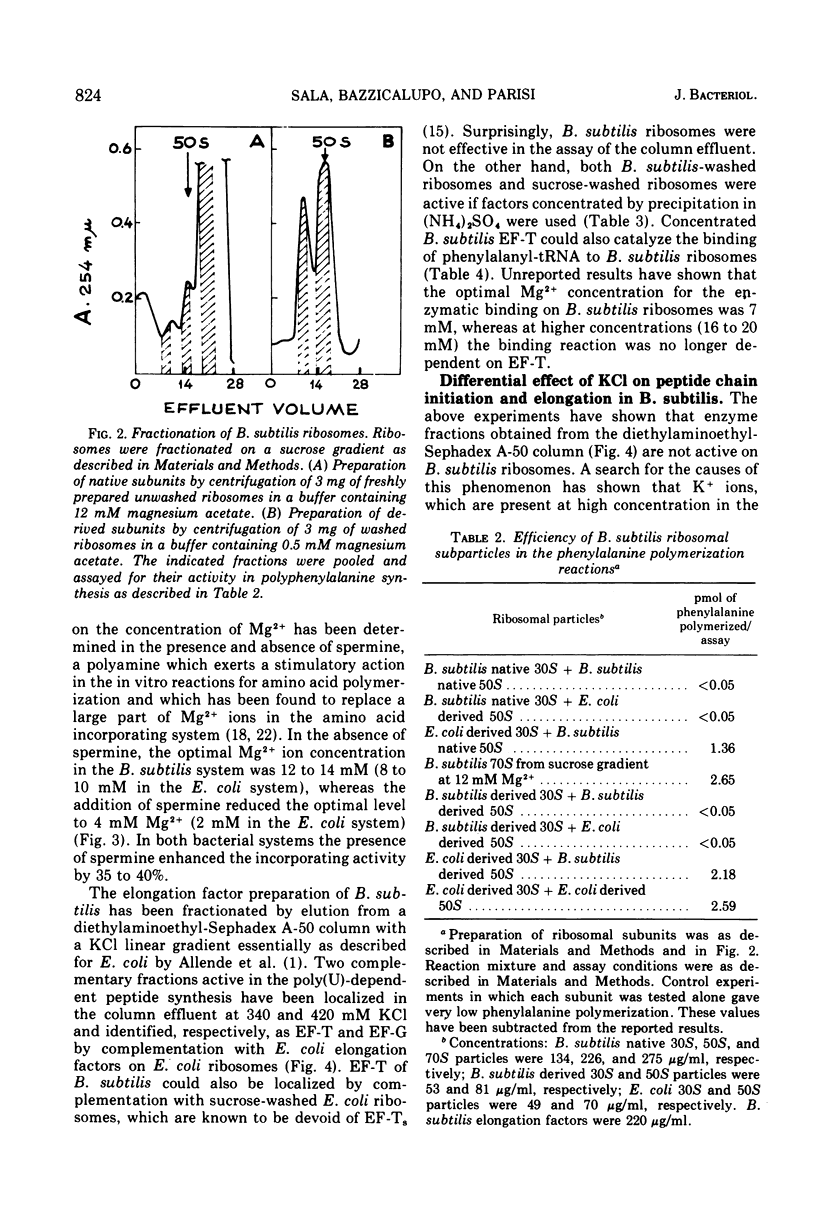
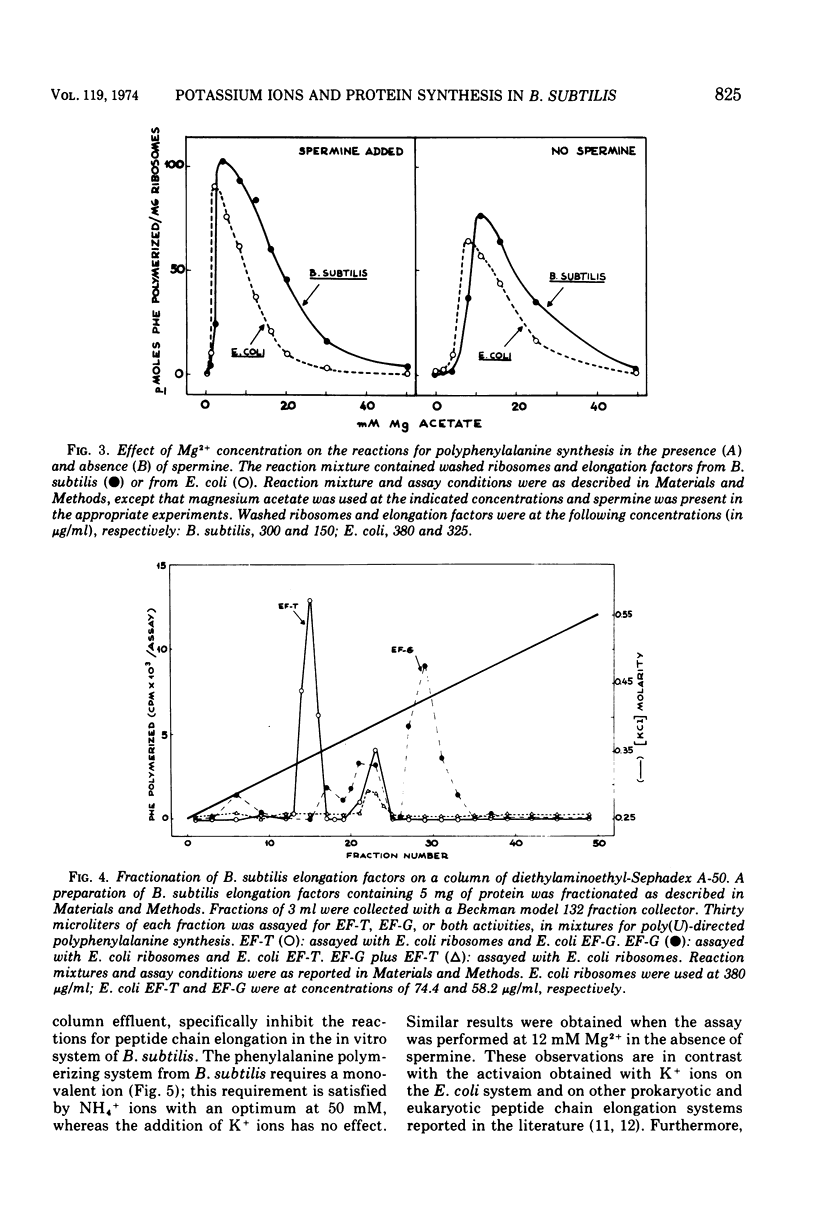
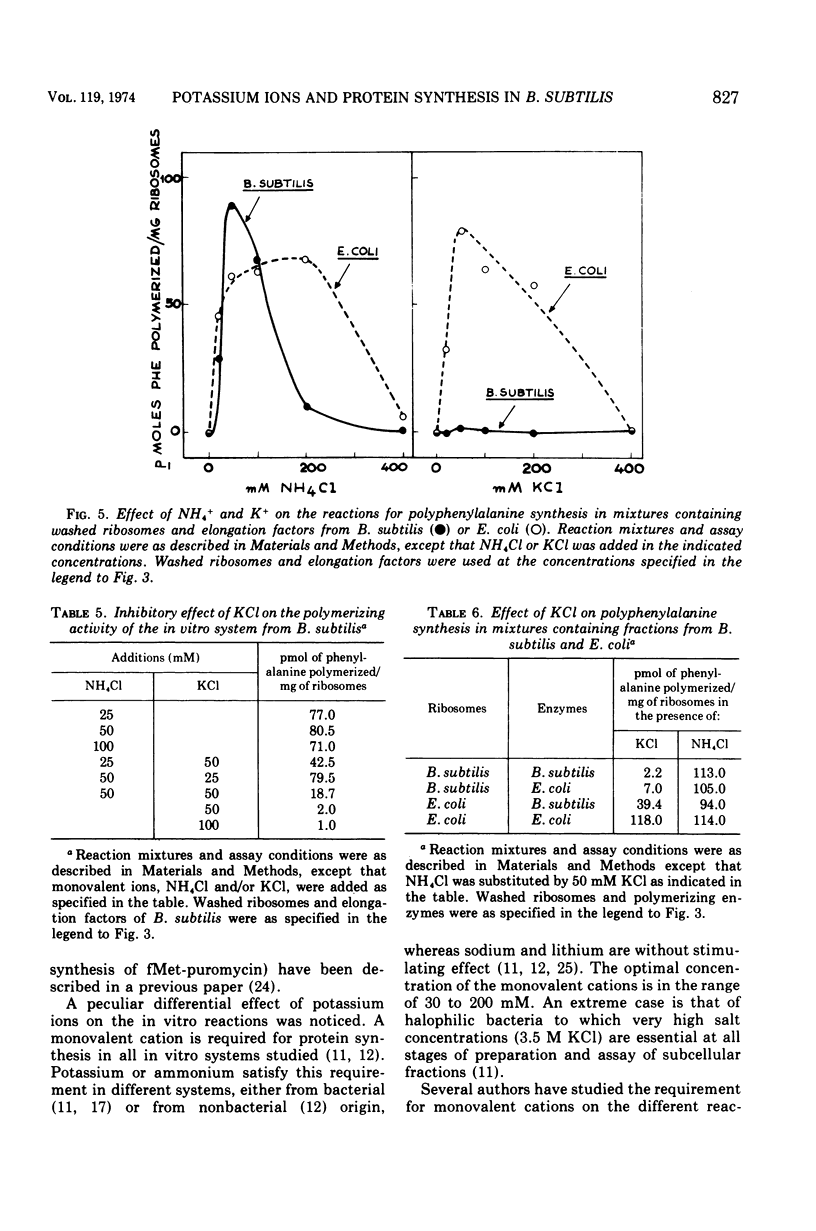
The preparation and fractionation of a highly active and stable in vitro protein-synthesizing system from Bacillus subtilis is described. Potassium satisfied the requirement for a monovalent ion when the initiation factor-dependent binding of formyl-methionyl-transfer ribonucleic acid and synthesis of formyl-methionyl-puromycin were assayed, whereas it inhibited the reactions for polyphenylalanine synthesis. On the other hand, the ammonium ion satisfied the requirement for all assayed reactions. The in vitro experimental evidence suggested that potassium is an inhibitor of one or a few specific reactions involved in peptide chain elongation in B. subtilis.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allende J. E., Seeds N. W., Conway T. W., Weissbach H. Guanosine triphosphate interaction with an amino acid polymerization factor from E. coli. Proc Natl Acad Sci U S A. 1967 Oct;58(4):1566–1573. doi: 10.1073/pnas.58.4.1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CONWAY T. W. ON THE ROLE OF AMMONIUM OR POTASSIUM ION IN AMINO ACID POLYMERIZATION. Proc Natl Acad Sci U S A. 1964 Jun;51:1216–1220. doi: 10.1073/pnas.51.6.1216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DAVIS B. D., MINGIOLI E. S. Mutants of Escherichia coli requiring methionine or vitamin B12. J Bacteriol. 1950 Jul;60(1):17–28. doi: 10.1128/jb.60.1.17-28.1950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubnau D., Goldthwaite C., Smith I., Marmur J. Genetic mapping in Bacillus subtilis. J Mol Biol. 1967 Jul 14;27(1):163–185. doi: 10.1016/0022-2836(67)90358-0. [DOI] [PubMed] [Google Scholar]
- Goldthwaite C., Dubnau D., Smith I. Genetic mapping of antibiotic resistance in markers Bacillus subtilis. Proc Natl Acad Sci U S A. 1970 Jan;65(1):96–103. doi: 10.1073/pnas.65.1.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harford N., Sueoka N. Chromosomal location of antibiotic resistance markers in Bacillus subtilis. J Mol Biol. 1970 Jul 28;51(2):267–286. doi: 10.1016/0022-2836(70)90142-7. [DOI] [PubMed] [Google Scholar]
- Hultin T. Factors influencing the puromycin-induced release of protein from liver ribosomes. Biochim Biophys Acta. 1966 Sep;123(3):561–573. doi: 10.1016/0005-2787(66)90223-1. [DOI] [PubMed] [Google Scholar]
- KAJI A., KAJI H., NOVELLI G. D. SOLUBLE AMINO ACID-INCORPORATING SYSTEM. I. PREPARATION OF THE SYSTEM AND NATURE OF THE REACTION. J Biol Chem. 1965 Mar;240:1185–1191. [PubMed] [Google Scholar]
- Levine H., Trindle M. R., Moldave K. Monovalent cation requirement for the aminoacyl transfer reaction in protein synthesis. Nature. 1966 Sep 17;211(5055):1302–1303. doi: 10.1038/2111302a0. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J., Lipmann F. Separation of three microbial amino acid polymerization factors. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1562–1566. doi: 10.1073/pnas.55.6.1562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas-Lenard J. Protein biosynthesis. Annu Rev Biochem. 1971;40:409–448. doi: 10.1146/annurev.bi.40.070171.002205. [DOI] [PubMed] [Google Scholar]
- Maden B. E., Monro R. E. Ribosome-catalyzed peptidyl transfer. Effects of cations and pH value. Eur J Biochem. 1968 Nov;6(2):309–316. doi: 10.1111/j.1432-1033.1968.tb00450.x. [DOI] [PubMed] [Google Scholar]
- Martin R. G., Ames B. N. THE EFFECT OF POLYAMINES AND OF POLY U SIZE ON PHENYLALANINE INCORPORATION. Proc Natl Acad Sci U S A. 1962 Dec;48(12):2171–2178. doi: 10.1073/pnas.48.12.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NIRENBERG M. W., MATTHAEI J. H. The dependence of cell-free protein synthesis in E. coli upon naturally occurring or synthetic polyribonucleotides. Proc Natl Acad Sci U S A. 1961 Oct 15;47:1588–1602. doi: 10.1073/pnas.47.10.1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parisi B., Milanesi G., Van Etten J. L., Perani A., Ciferri O. Species specificity in protein synthesis. J Mol Biol. 1967 Sep 14;28(2):295–309. doi: 10.1016/s0022-2836(67)80011-1. [DOI] [PubMed] [Google Scholar]
- Ranu R. S., Kaji A. Evidence for specific inhibition of translocation of aminoacyl-transfer ribonucleic acid by the pretreatment of ribosomes of Bacillus subtilis with p-chloromercuribenzoate. J Bacteriol. 1972 Oct;112(1):188–194. doi: 10.1128/jb.112.1.188-194.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPYRIDES G. J. THE EFFECT OF UNIVALENT CATIONS ON THE BINDING OF SRNA TO THE TEMPLATE-RIBOSOME COMPLEX. Proc Natl Acad Sci U S A. 1964 Jun;51:1220–1226. doi: 10.1073/pnas.51.6.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sala F., Küntzel H. Peptide chain initiation in homologous and heterologous systems from mitochondria and bacteria. Eur J Biochem. 1970 Aug;15(2):280–286. doi: 10.1111/j.1432-1033.1970.tb01005.x. [DOI] [PubMed] [Google Scholar]
- Takeda M., Lipmann F. Comparison of amino Acid polymerization in B. Subtilis and e. Coli cell-free systems; hybridization of their ribosomes. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1875–1882. doi: 10.1073/pnas.56.6.1875. [DOI] [PMC free article] [PubMed] [Google Scholar]



