Abstract
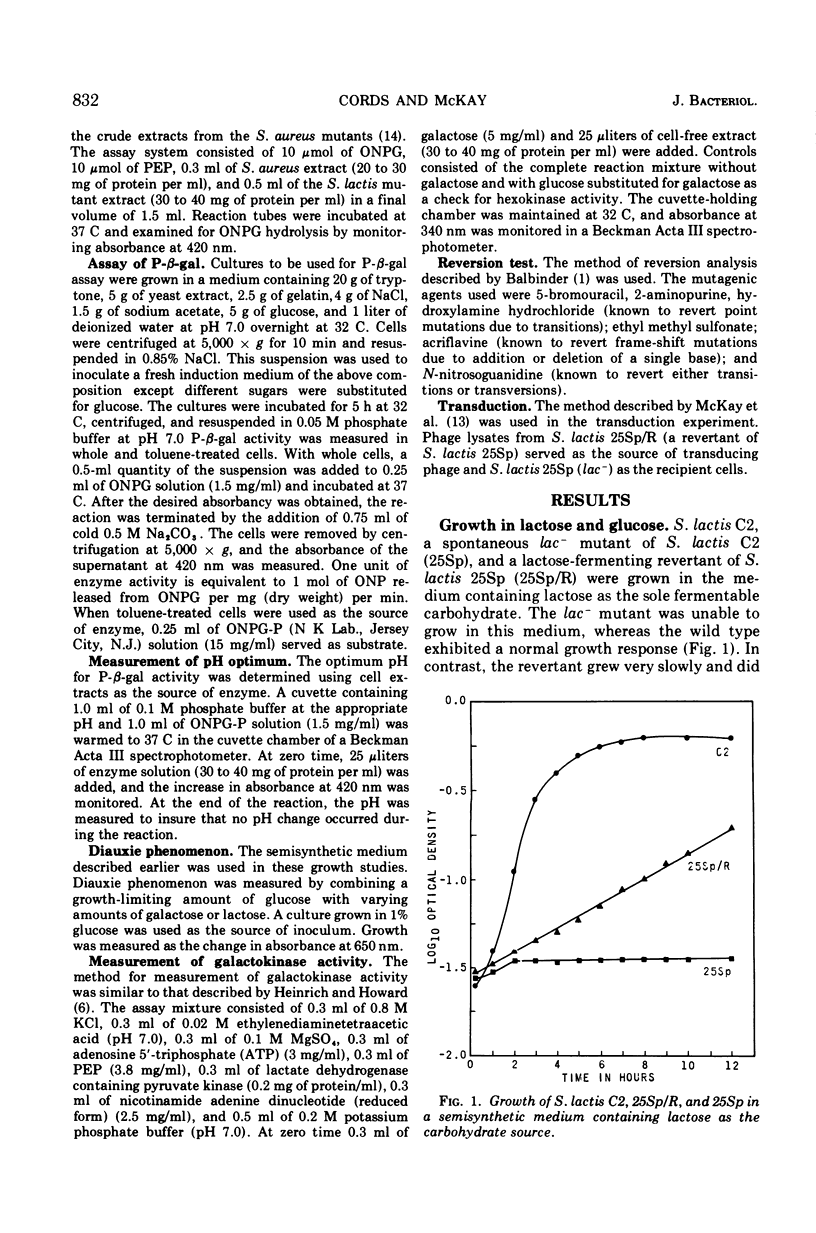
Partial lactose-fermenting revertants from lactose-negative (lac−) mutants of Streptococcus lactis C2 appeared on a lawn of lac− cells after 3 to 5 days of incubation at 25 C. The revertants grew slowly on lactose with a growth response similar to that for cryptic cells. In contrast to lac+S. lactis C2, the revertants were defective in the accumulation of [14C]thiomethyl-β-d-galactoside, indicating that they were devoid of a transport system. Hydrolysis of o-nitrophenyl-β-d-galactoside-6-phosphate by toluene-treated cells confirmed the presence of phospho-β-d-galactosidase (P-β-gal) in the revertant. However, this enzyme was induced only when the cells were grown in the presence of lactose; galactose was not an inducer. In lac+S. lactis C2, enzyme induction occurred in lactose- or galactose-grown cells. The revertants were defective in EII-lactose and FIII-lactose of the phosphoenolpyruvate-dependent phosphotransferase system. Galactokinase activity was detected in cell extracts of lac+S. lactis C2, but the activity was 9 to 13 times higher in extracts from the revertant and lac−, respectively. This suggested that the lac− and the revertants use the Leloir pathway for galactose metabolism and that galactose-1-phosphate rather than galactose-6-phosphate was being formed. This may explain why lactose, but not galactose, induced P-β-gal in the revertants. Because the revertant was unable to form galactose-6-phosphate, induction could not occur. This compound would be formed on hydrolysis of lactose phosphate. The data also indicate that galactose-6-phosphate may serve not only as an inducer of the lactose genes in S. lactis C2, but also as a repressor of the Leloir pathway for galactose metabolism.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balbinder E. The Fine Structure of the Loci Tryc and Tryd of Salmonella Typhimurium. II. Studies of Reversion Patterns and the Behavior of Specific Alleles during Recombination. Genetics. 1962 May;47(5):545–559. doi: 10.1093/genetics/47.5.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bissett D. L., Anderson R. L. Lactose and D-galactose metabolism in group N streptococci: presence of enzymes for both the D-galactose 1-phosphate and D-tagatose 6-phosphate pathways. J Bacteriol. 1974 Jan;117(1):318–320. doi: 10.1128/jb.117.1.318-320.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell J. H., Lengyel J. A., Langridge J. Evolution of a second gene for beta-galactosidase in Escherichia coli. Proc Natl Acad Sci U S A. 1973 Jun;70(6):1841–1845. doi: 10.1073/pnas.70.6.1841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demko G. M., Blanton S. J., Benoit R. E. Heterofermentative carbohydrate metabolism of lactose-impaired mutants of Streptococcus lactis. J Bacteriol. 1972 Dec;112(3):1335–1345. doi: 10.1128/jb.112.3.1335-1345.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERZENBERG L. A. Studies on the induction of beta-galactosidase in a cryptic strain of Escherichia coli. Biochim Biophys Acta. 1959 Feb;31(2):525–538. doi: 10.1016/0006-3002(59)90029-0. [DOI] [PubMed] [Google Scholar]
- HIRSCH A. Growth and nisin production of a strain of Streptococcus lactis. J Gen Microbiol. 1951 Feb;5(1):208–221. doi: 10.1099/00221287-5-1-208. [DOI] [PubMed] [Google Scholar]
- Hengstenberg W., Penberthy W. K., Hill K. L., Morse M. L. Phosphotransferase system of Staphylococcus aureus: its requirement for the accumulation and metabolism of galactosides. J Bacteriol. 1969 Aug;99(2):383–388. doi: 10.1128/jb.99.2.383-388.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R., Molskness T., Sandine W. E., Elliker P. R. Carbohydrate metabolism in lactic streptococci: fate of galactose supplied in free or disaccharide form. Appl Microbiol. 1973 Dec;26(6):951–958. doi: 10.1128/am.26.6.951-958.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Baldwin K. A., Zottola E. A. Loss of lactose metabolism in lactic streptococci. Appl Microbiol. 1972 Jun;23(6):1090–1096. doi: 10.1128/am.23.6.1090-1096.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Cords B. R., Baldwin K. A. Transduction of lactose metabolism in Streptococcus lactis C2. J Bacteriol. 1973 Sep;115(3):810–815. doi: 10.1128/jb.115.3.810-815.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molskness T. A., Lee D. R., Sandine W. E., Elliker P. R. -D-phosphogalactoside galactohydrolase of lactic streptococci. Appl Microbiol. 1973 Mar;25(3):373–380. doi: 10.1128/am.25.3.373-380.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morse M. L., Hill K. L., Egan J. B., Hengstenberg W. Metabolism of lactose by Staphylococcus aureus and its genetic basis. J Bacteriol. 1968 Jun;95(6):2270–2274. doi: 10.1128/jb.95.6.2270-2274.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reeve E. C., Braithwaite J. A. The lactose system in Klebsiella aerogenes V9A. 2. Galactoside permeases which accumulate lactose or melibiose. Genet Res. 1973 Jun;21(3):273–285. doi: 10.1017/s001667230001346x. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Roseman S. Sugar transport. VII. Lactose transport in Staphylococcus aureus. J Biol Chem. 1973 Feb 10;248(3):966–974. [PubMed] [Google Scholar]
- Warren R. A. Lactose-utilizing mutants of lac deletion strains of Escherichia coli. Can J Microbiol. 1972 Sep;18(9):1439–1444. doi: 10.1139/m72-221. [DOI] [PubMed] [Google Scholar]


