Abstract
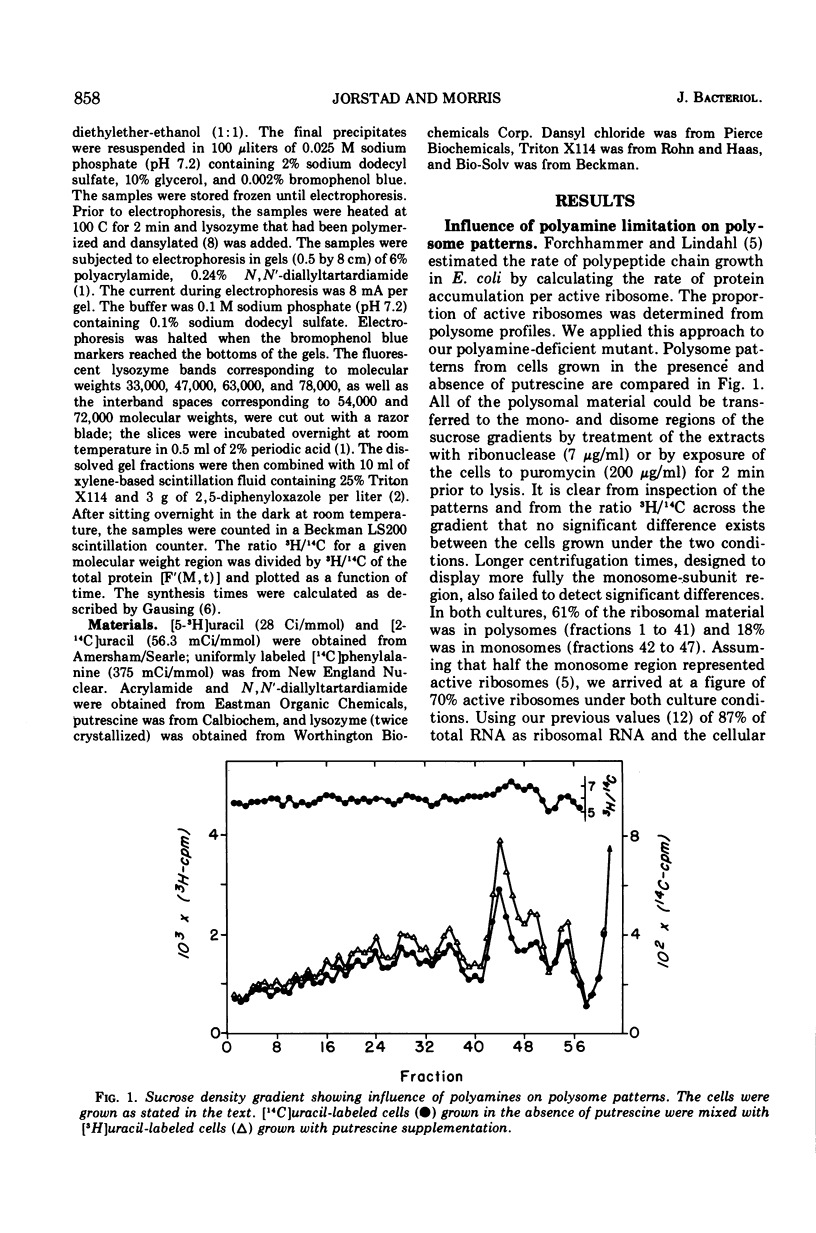
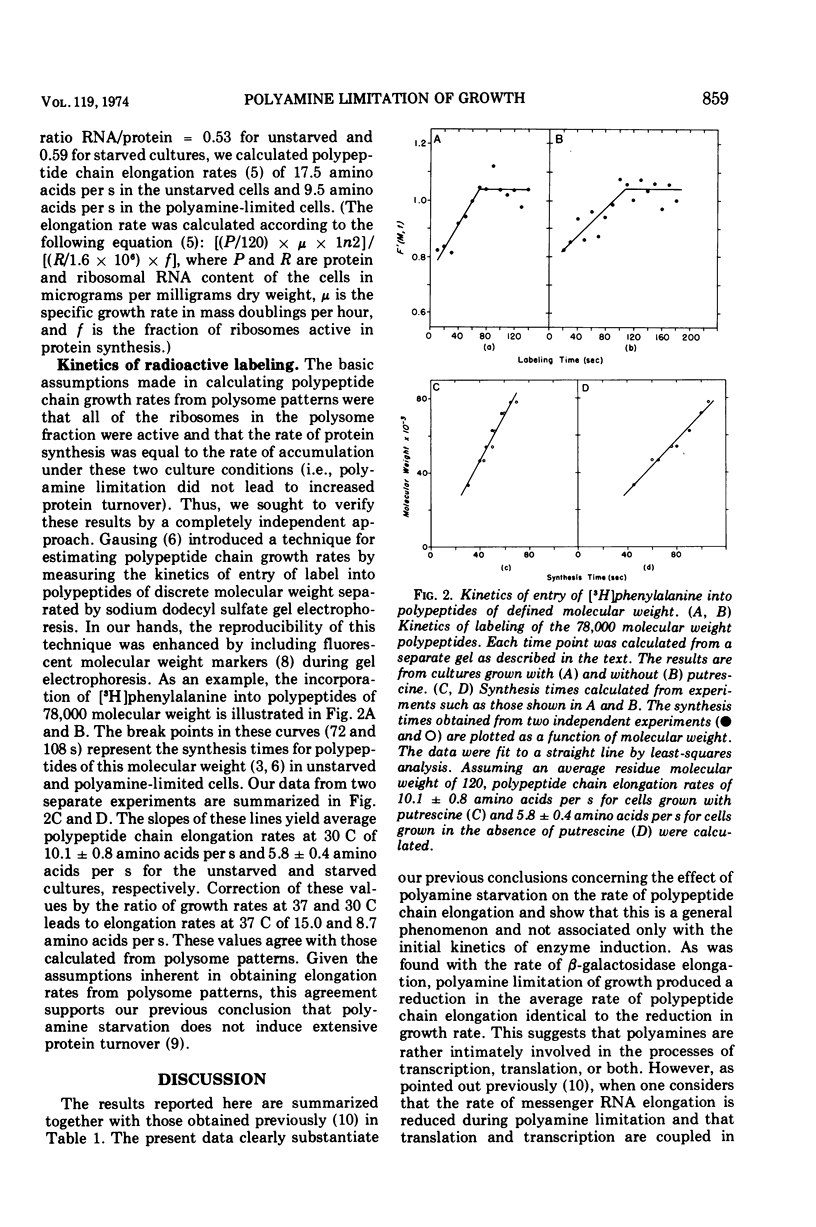
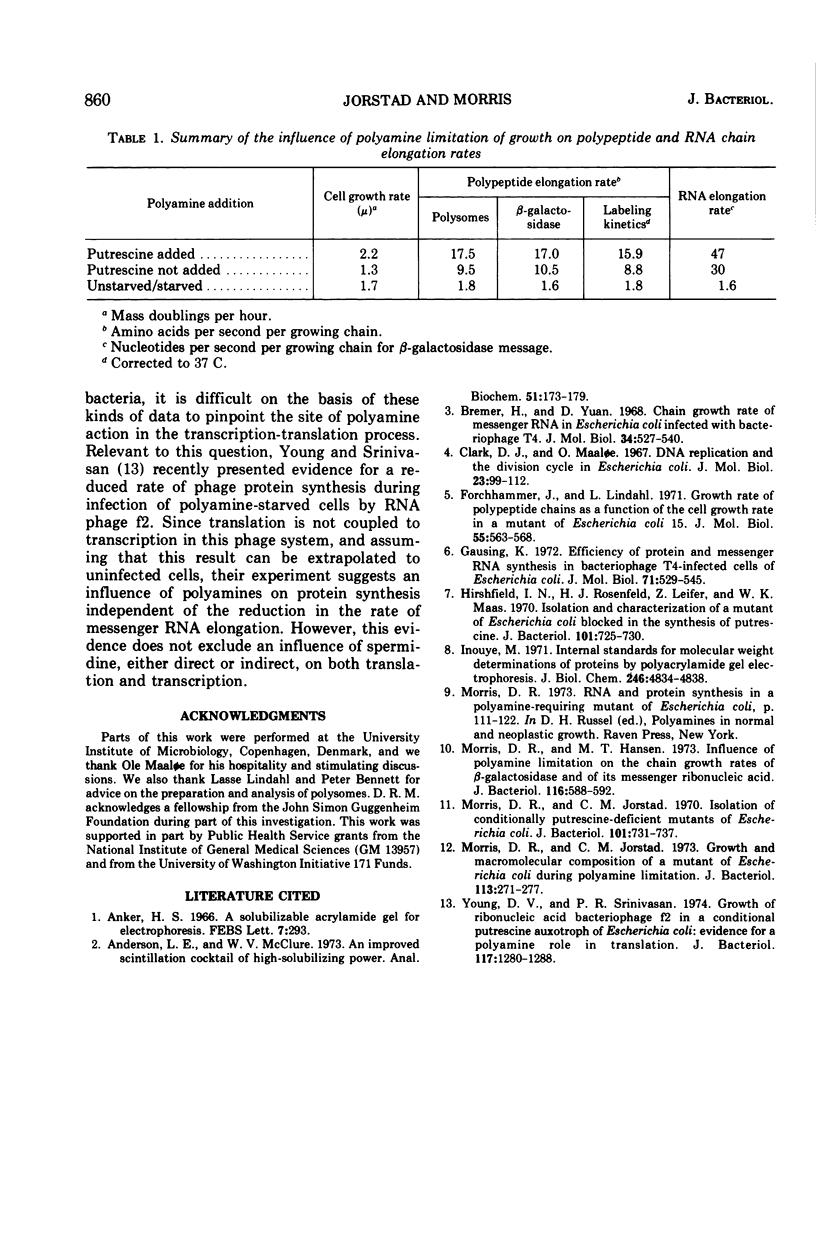
The rate of polypeptide chain elongation during steady-state, polyamine-limited growth of a mutant of Escherichia coli was measured by two independent techniques. Analysis of polysome patterns gave values of 17.5 and 9.5 amino acids per s at 37 C in unstarved and polyamine-limited cells, respectively. From the kinetics of entry of labeled amino acids into polypeptides of defined molecular weights, values at 30 C of 10.1 and 5.8 amino acids per s were obtained for unstarved and polyamine-limited cultures, respectively. Correction of these values to 37 C resulted in rates of 15.0 and 8.7 amino acids per s. These results support the previous conclusion, based on the kinetics of β-galactosidase induction, that polyamine starvation decreases the rate of protein synthesis by limiting the velocity of polypeptide chain elongation.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson L. E., McClure W. O. An improved scintillation cocktail of high-solubilizing power. Anal Biochem. 1973 Jan;51(1):173–179. doi: 10.1016/0003-2697(73)90465-x. [DOI] [PubMed] [Google Scholar]
- Anker H. S. A solubilizable acrylamide gel for electrophoresis. FEBS Lett. 1970 Apr 16;7(3):293–293. doi: 10.1016/0014-5793(70)80185-5. [DOI] [PubMed] [Google Scholar]
- Bremer H., Yuan D. Chain growth rate of messenger RNA in Escherichia coli infected with bacteriophage T4. J Mol Biol. 1968 Jun 28;34(3):527–540. doi: 10.1016/0022-2836(68)90178-2. [DOI] [PubMed] [Google Scholar]
- Forchhammer J., Lindahl L. Growth rate of polypeptide chains as a function of the cell growth rate in a mutant of Escherichia coli 15. J Mol Biol. 1971 Feb 14;55(3):563–568. doi: 10.1016/0022-2836(71)90337-8. [DOI] [PubMed] [Google Scholar]
- Gausing K. Efficiency of protein and messenger RNA synthesis in bacteriophage T4-infected cells of Escherichia coli. J Mol Biol. 1972 Nov 28;71(3):529–545. doi: 10.1016/s0022-2836(72)80021-4. [DOI] [PubMed] [Google Scholar]
- Hirshfield I. N., Rosenfeld H. J., Leifer Z., Maas W. K. Isolation and characterization of a mutant of Escherichia coli blocked in the synthesis of putrescine. J Bacteriol. 1970 Mar;101(3):725–730. doi: 10.1128/jb.101.3.725-730.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inouye M. Internal standards for molecular weight determinations of proteins by polyacrylamide gel electrophoresis. Applications to envelope proteins of Escherichia coli. J Biol Chem. 1971 Aug 10;246(15):4834–4838. [PubMed] [Google Scholar]
- Morris D. R., Hansen M. T. Influence of polyamine limitation on the chain growth rates of beta-galactosidase and of its messenger ribonucleic acid. J Bacteriol. 1973 Nov;116(2):588–592. doi: 10.1128/jb.116.2.588-592.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Jorstad C. M. Growth and macromolecular composition of a mutant of Escherichia coli during polyamine limitation. J Bacteriol. 1973 Jan;113(1):271–277. doi: 10.1128/jb.113.1.271-277.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morris D. R., Jorstad C. M. Isolation of conditionally putrescine-deficient mutants of Escherichia coli. J Bacteriol. 1970 Mar;101(3):731–737. doi: 10.1128/jb.101.3.731-737.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young D. V., Srinivasan P. R. Growth of ribonucleic acid bacteriophage f2 in a conditional putrescine auxotroph of Escherichia coli: evidence for a polyamine role in translation. J Bacteriol. 1974 Mar;117(3):1280–1288. doi: 10.1128/jb.117.3.1280-1288.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]



