Abstract
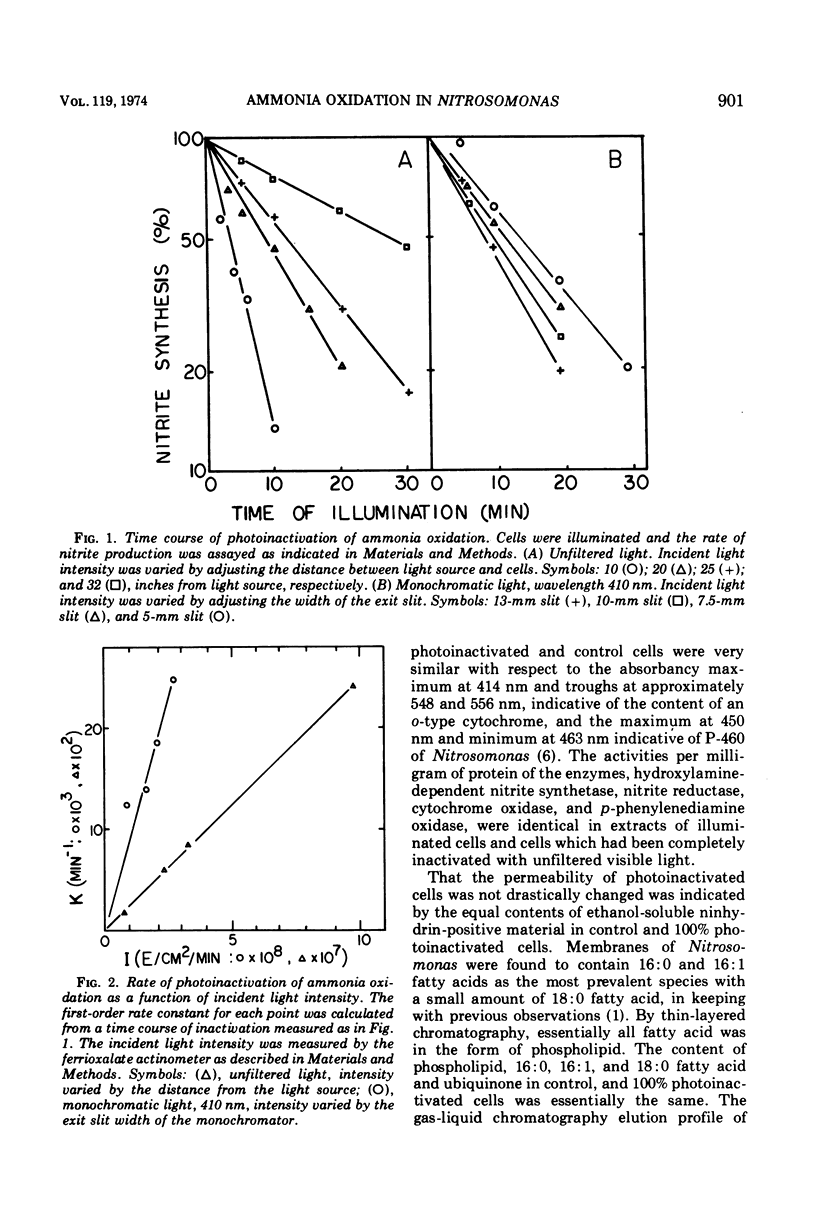
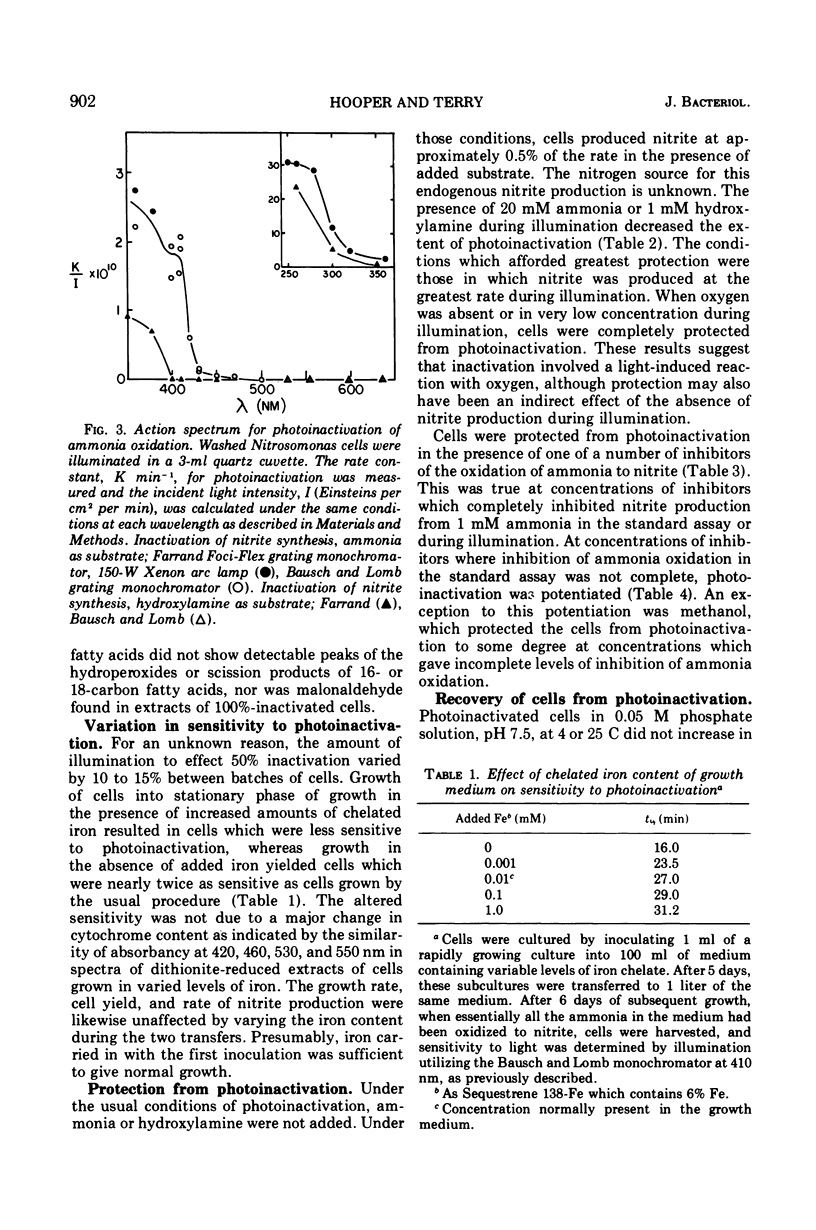
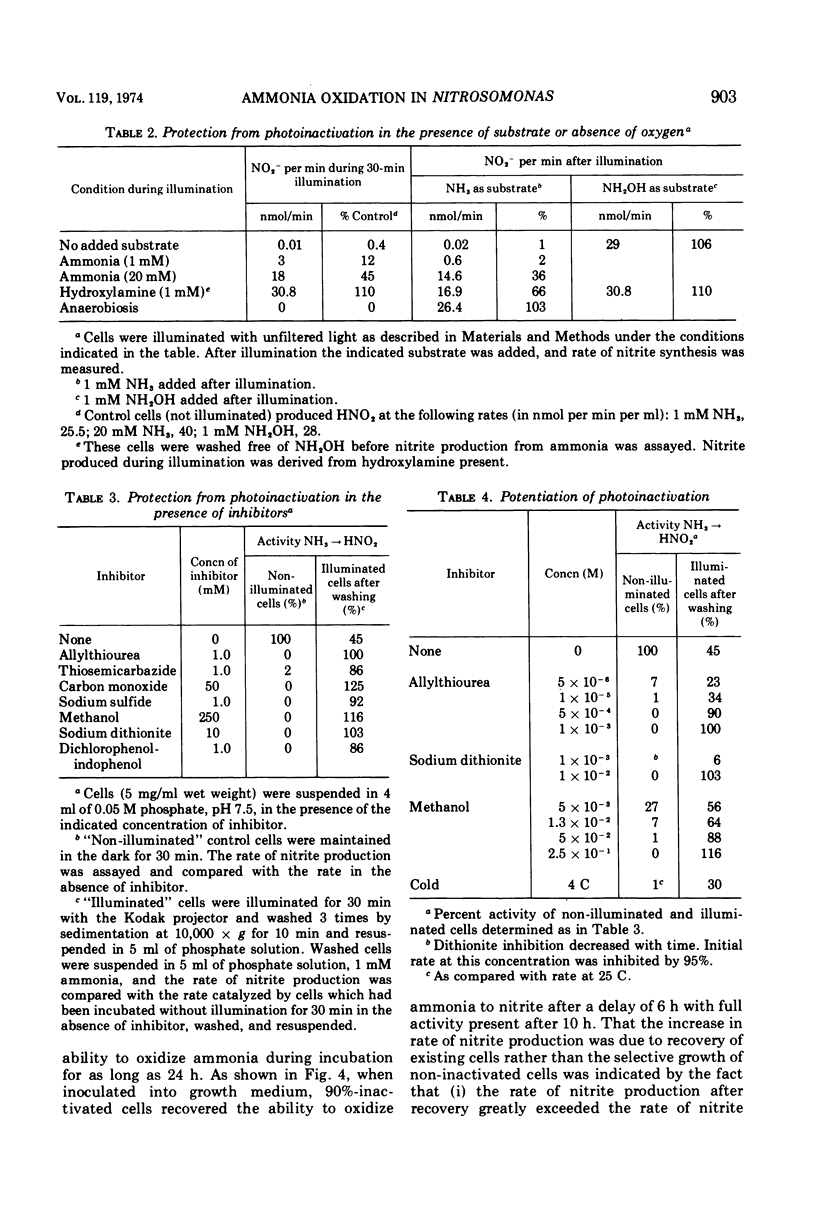
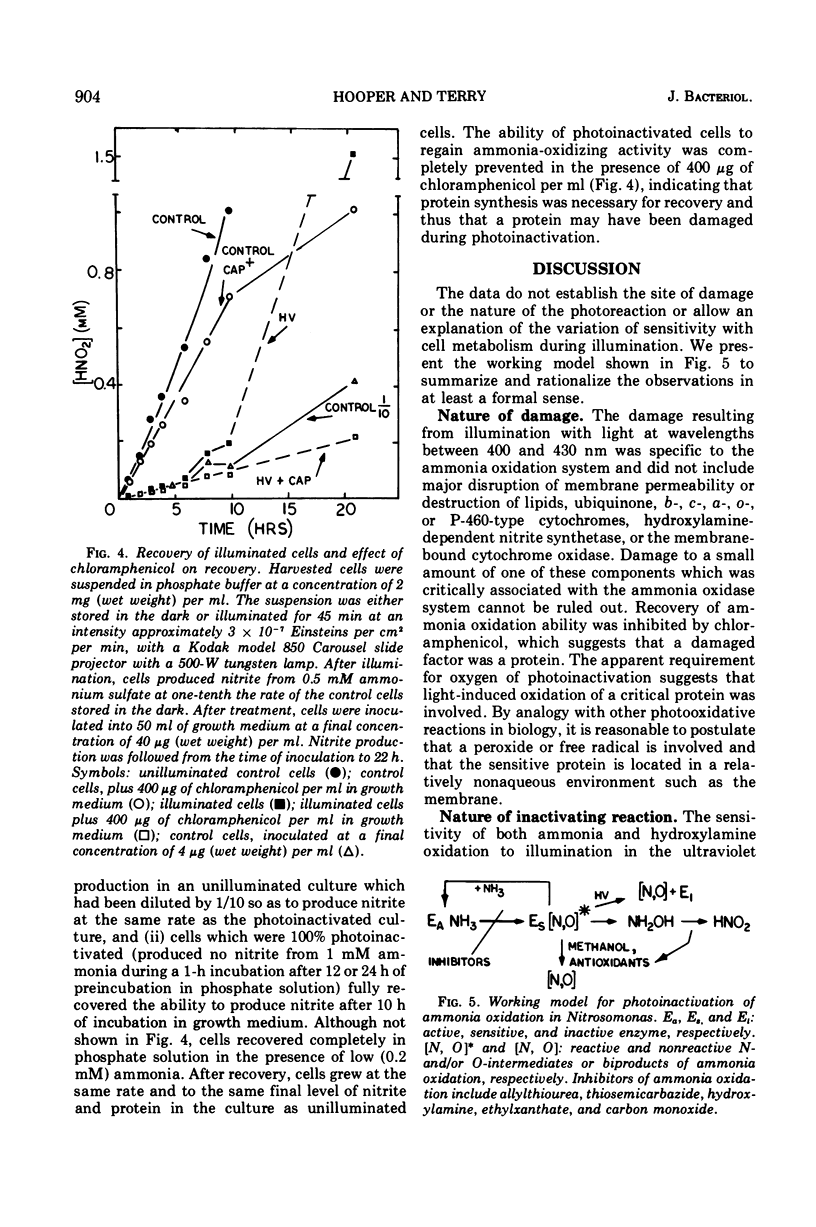
Photoinactivation of ammonia oxidation in cells of Nitrosomonas was shown to follow first-order kinetics with a rate constant proportional to incident light intensity. The action spectrum for photoinactivation consisted of a broad peak in the ultraviolet range, where both hydroxylamine and ammonia oxidation were affected, and a shoulder at approximately 410 nm where only ammonia oxidation was affected. In photoinactivated cells, hydroxylamine but not ammonia was oxidized to nitrite and hydroxylamine but not ammonia caused reduction of cytochromes in vivo. The amount per cell of the following constituents was not measurably altered by photoinactivation: cytochromes b, c, a, and P460; ubiquinone; phospholipid; free amino acids; hydroxylamine-dependent nitrite synthetase; nitrite reductase; p-phenylenediamine oxidase; and cytochrome c oxidase. Malonaldehyde or lipid peroxides were not detected in photoinactivated cells. Photoinactivation was prevented (i) under anaerobic conditions, (ii) in the presence of methanol, allylthiourea, thiosemicarbazide, hydroxylamine, ethylxanthate, or CO at concentrations wich caused 100% inhibition of ammonia oxidation, and (iii) at concentrations of ammonia or hydroxylamine which gave a rapid rate of nitrite production. Recovery of ammonia oxidation activity in 90% inactivated cells took place in 6 h, required an energy and/or nitrogen source, and was inhibited by 400 μg of chloramphenicol per ml.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Blumer M., Chase T., Watson S. W. Fatty acids in the lipids of marine and terrestrial nitrifying bacteria. J Bacteriol. 1969 Aug;99(2):366–370. doi: 10.1128/jb.99.2.366-370.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D. Effect of near-ultraviolet light on the respiratory chain of Escherichia coli. Can J Biochem. 1971 May;49(5):492–495. doi: 10.1139/o71-073. [DOI] [PubMed] [Google Scholar]
- Burchard R. P., Dworkin M. Light-induced lysis and carotenogenesis in Myxococcus xanthus. J Bacteriol. 1966 Feb;91(2):535–545. doi: 10.1128/jb.91.2.535-545.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson R. H., Hooper A. B. Preliminary characterization of a variant co-binding heme protein from Nitrosomonas. Biochim Biophys Acta. 1972 Aug 17;275(2):231–244. doi: 10.1016/0005-2728(72)90044-8. [DOI] [PubMed] [Google Scholar]
- Erickson R. H., Hooper A. B., Terry K. R. Solubilization and purification of cytochrome 1 from Nitrosomonas. Biochim Biophys Acta. 1972;283(1):155–166. doi: 10.1016/0005-2728(72)90107-7. [DOI] [PubMed] [Google Scholar]
- Hooper A. B. A nitrite-reducing enzyme from Nitrosomonas europaea. Preliminary characterization with hydroxylamine ad electron donor. Biochim Biophys Acta. 1968 Jul 16;162(1):49–65. doi: 10.1016/0005-2728(68)90213-2. [DOI] [PubMed] [Google Scholar]
- Hooper A. B., Erickson R. H., Terry K. R. Electron transport systems of Nitrosomonas: isolation of a membrane-envelope fraction. J Bacteriol. 1972 Apr;110(1):430–438. doi: 10.1128/jb.110.1.430-438.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. B. Lag phase of ammonia oxidation by resting cells of Nitrosomonas eruopaea. J Bacteriol. 1969 Feb;97(2):968–969. doi: 10.1128/jb.97.2.968-969.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hooper A. B., Terry K. R. Specific inhibitors of ammonia oxidation in Nitrosomonas. J Bacteriol. 1973 Aug;115(2):480–485. doi: 10.1128/jb.115.2.480-485.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamola A. A., Yamane T., Trozzolo A. M. Cholesterol hydroperoxide formation in red cell membranes and photohemolysis in erythropoietic protoporphyria. Science. 1973 Mar 16;179(4078):1131–1133. doi: 10.1126/science.179.4078.1131. [DOI] [PubMed] [Google Scholar]



