Abstract
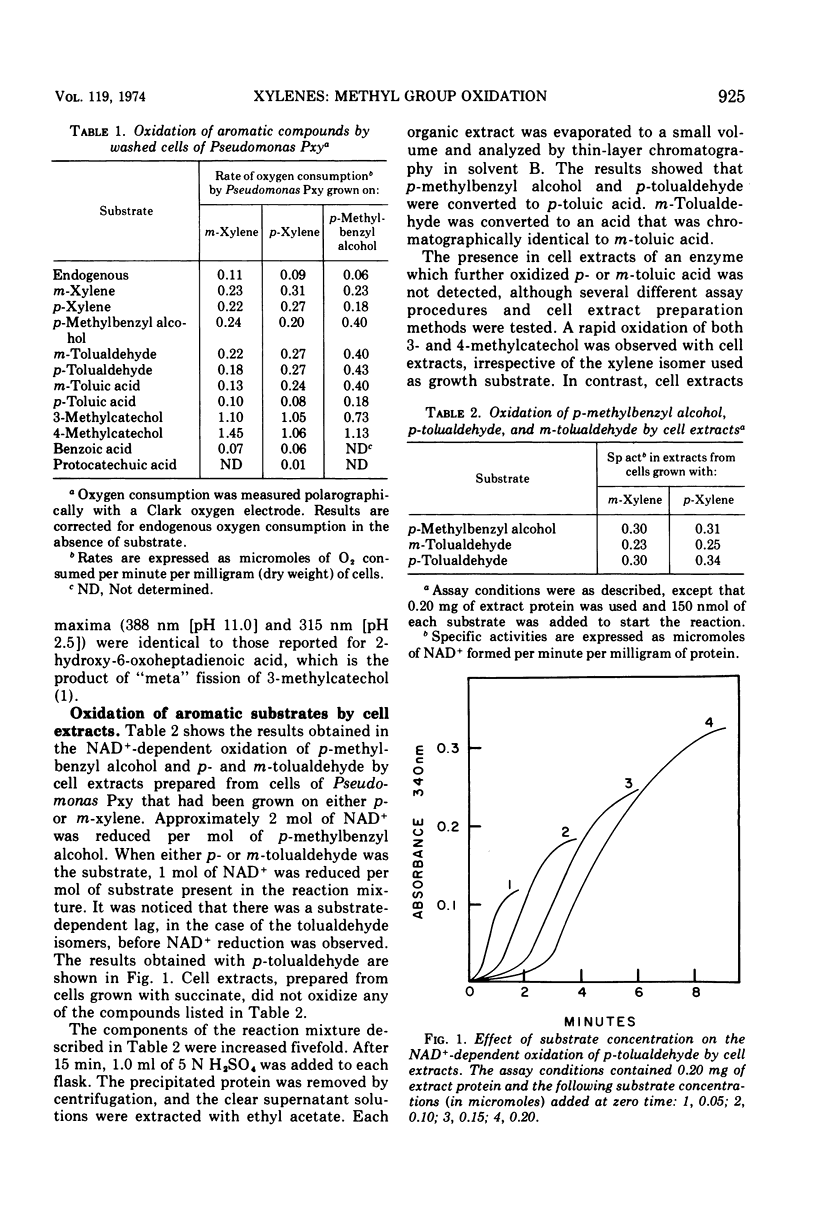
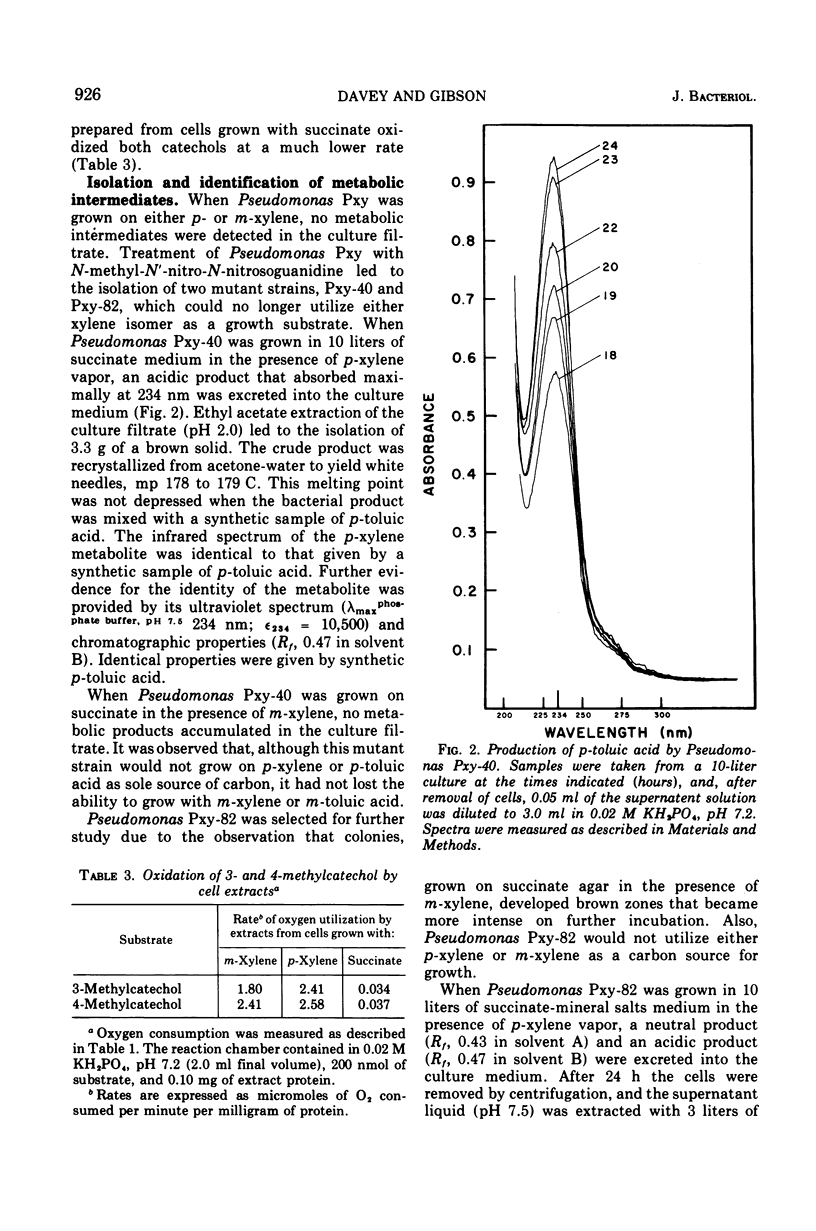
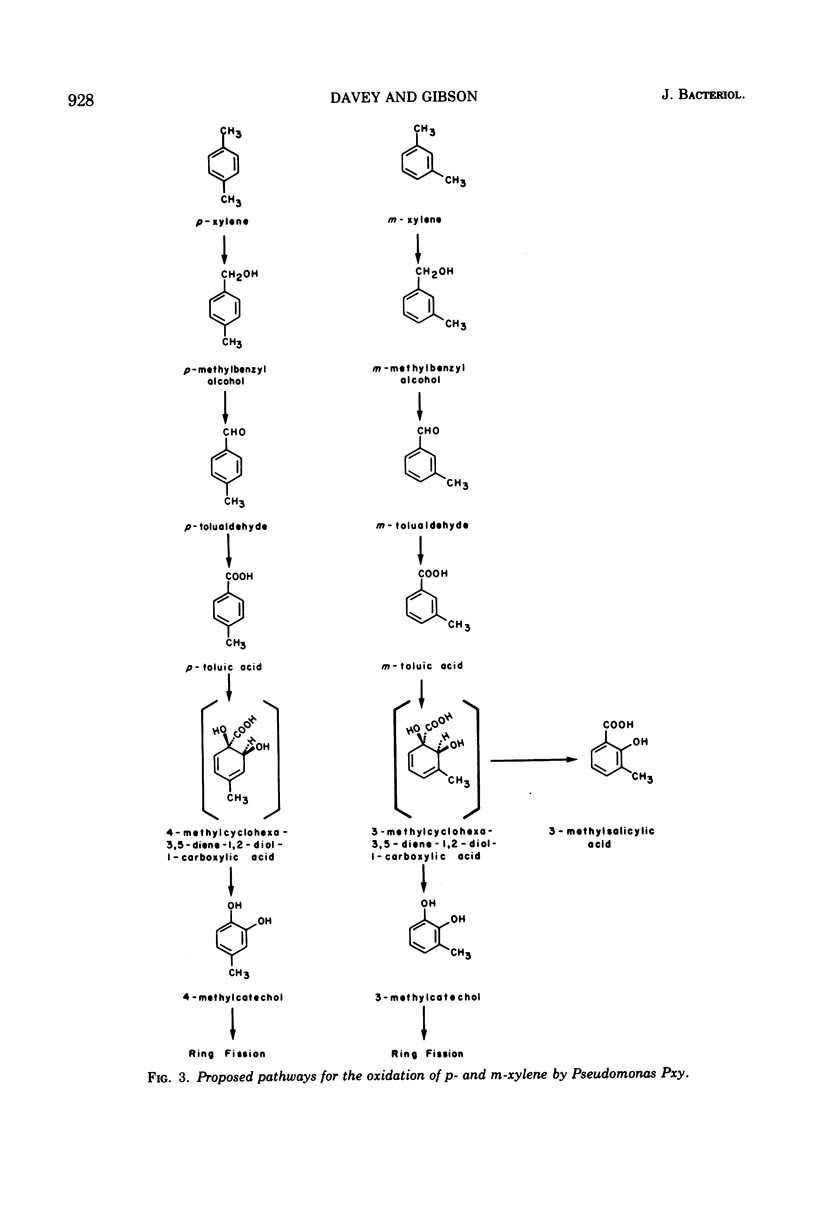
Pseudomonas Pxy was isolated on p-xylene as sole source of carbon and energy. Substrates that supported growth were toluene, p-methylbenzyl alcohol, p-tolualdehyde, p-toluic acid, and the analogous m-methyl derivatives, including m-xylene. Cell extracts prepared from Pseudomonas Pxy after growth with either p-xylene or m-xylene oxidized the p- and m-isomers of tolualdehyde as well as p-methylbenzyl alcohol. The same cell extracts also catalyzed a “meta” fission of both 3- and 4-methylcatechol. Treatment of Pseudomonas Pxy with N-methyl-N′-nitro-N-nitrosoguanidine led to the isolation of two mutant strains. Pseudomonas Pxy-40, when grown on succinate in the presence of p-xylene, accumulated p-toluic acid in the culture medium. Under the same conditions Pseudomonas Pxy-82 accumulated p-toluic acid and also 4-methylcatechol. When Pseudomonas Pxy-82 was grown on succinate in the presence of m-xylene, 3-methylcatechol and 3-methylsalicylic acid were excreted into the culture medium. A pathway is proposed for the initial reactions utilized by Pseudomonas Pxy to oxidize p- and m-xylene.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bayly R. C., Dagley S., Gibson D. T. The metabolism of cresols by species of Pseudomonas. Biochem J. 1966 Nov;101(2):293–301. doi: 10.1042/bj1010293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis R. S., Hossler F. E., Stone R. W. Metabolism of p- and m-xylene by species of Pseudomonas. Can J Microbiol. 1968 Sep;14(9):1005–1009. doi: 10.1139/m68-166. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Gschwendt B., Yeh W. K., Kobal V. M. Initial reactions in the oxidation of ethylbenzene by Pseudomonas putida. Biochemistry. 1973 Apr 10;12(8):1520–1528. doi: 10.1021/bi00732a008. [DOI] [PubMed] [Google Scholar]
- Gibson D. T., Mahadevan V., Davey J. F. Bacterial metabolism of para- and meta-xylene: oxidation of the aromatic ring. J Bacteriol. 1974 Sep;119(3):930–936. doi: 10.1128/jb.119.3.930-936.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Murray K., Duggleby C. J., Sala-Trepat J. M., Williams P. A. The metabolism of benzoate and methylbenzoates via the meta-cleavage pathway by Pseudomonas arvilla mt-2. Eur J Biochem. 1972 Jul 24;28(3):301–310. doi: 10.1111/j.1432-1033.1972.tb01914.x. [DOI] [PubMed] [Google Scholar]
- Ornston L. N. The conversion of catechol and protocatechuate to beta-ketoadipate by Pseudomonas putida. IV. Regulation. J Biol Chem. 1966 Aug 25;241(16):3800–3810. [PubMed] [Google Scholar]
- Reiner A. M., Hegeman G. D. Metabolism of benzoic acid by bacteria. Accumulation of (-)-3,5-cyclohexadiene-1,2-diol-1-carboxylic acid by mutant strain of Alcaligenes eutrophus. Biochemistry. 1971 Jun 22;10(13):2530–2536. doi: 10.1021/bi00789a017. [DOI] [PubMed] [Google Scholar]
- Ribbons D. W. Metabolism of omicron-cresol by Pseudomonas aeruginosa strain T1. J Gen Microbiol. 1966 Aug;44(2):221–231. doi: 10.1099/00221287-44-2-221. [DOI] [PubMed] [Google Scholar]
- Stanier R. Y., Palleroni N. J., Doudoroff M. The aerobic pseudomonads: a taxonomic study. J Gen Microbiol. 1966 May;43(2):159–271. doi: 10.1099/00221287-43-2-159. [DOI] [PubMed] [Google Scholar]


