Abstract
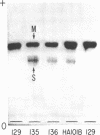
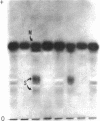
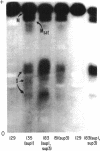
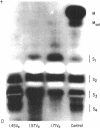
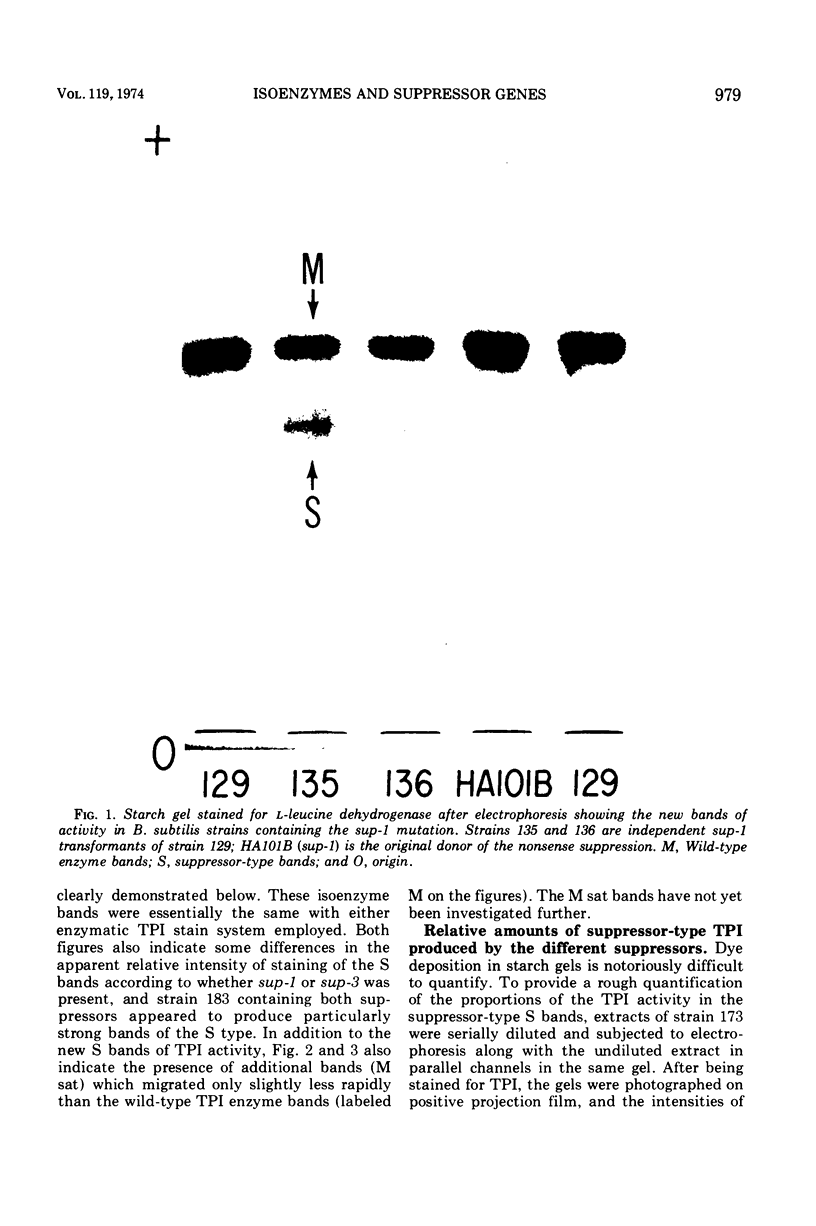
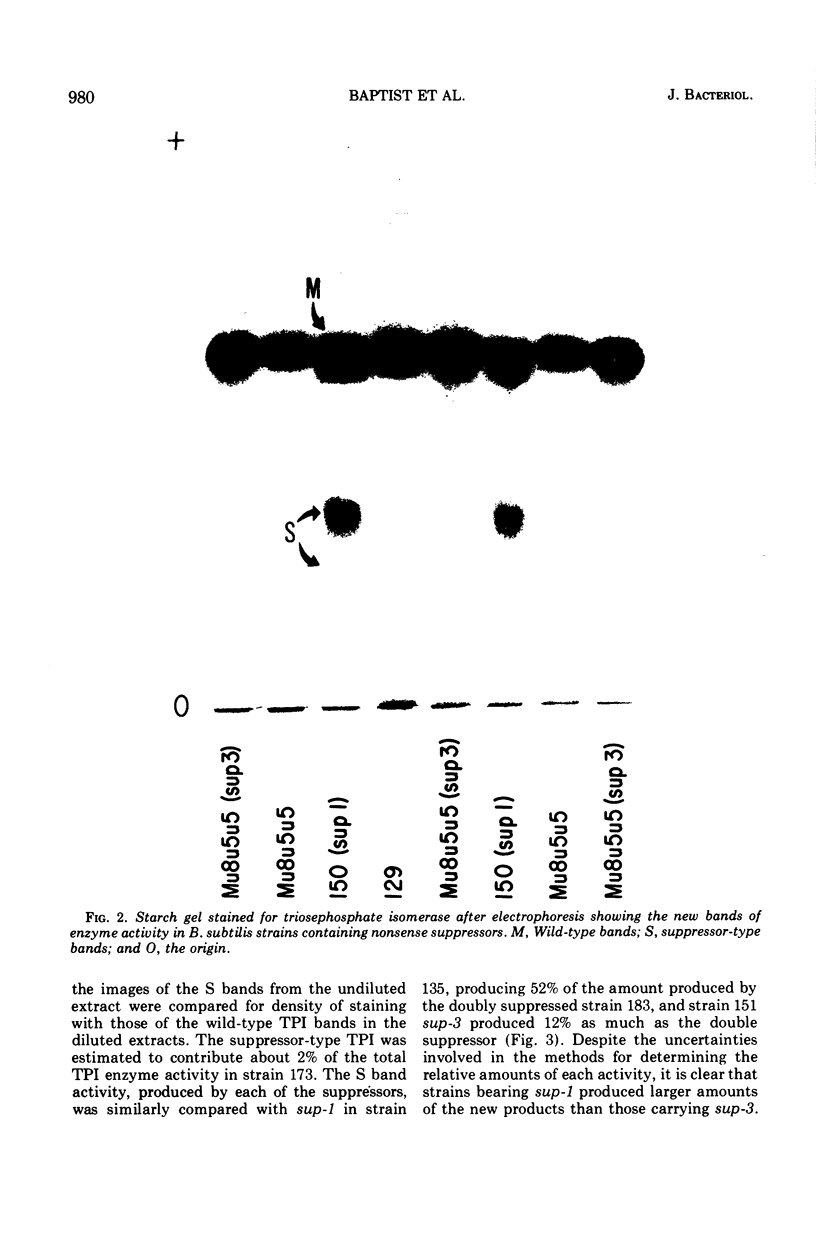
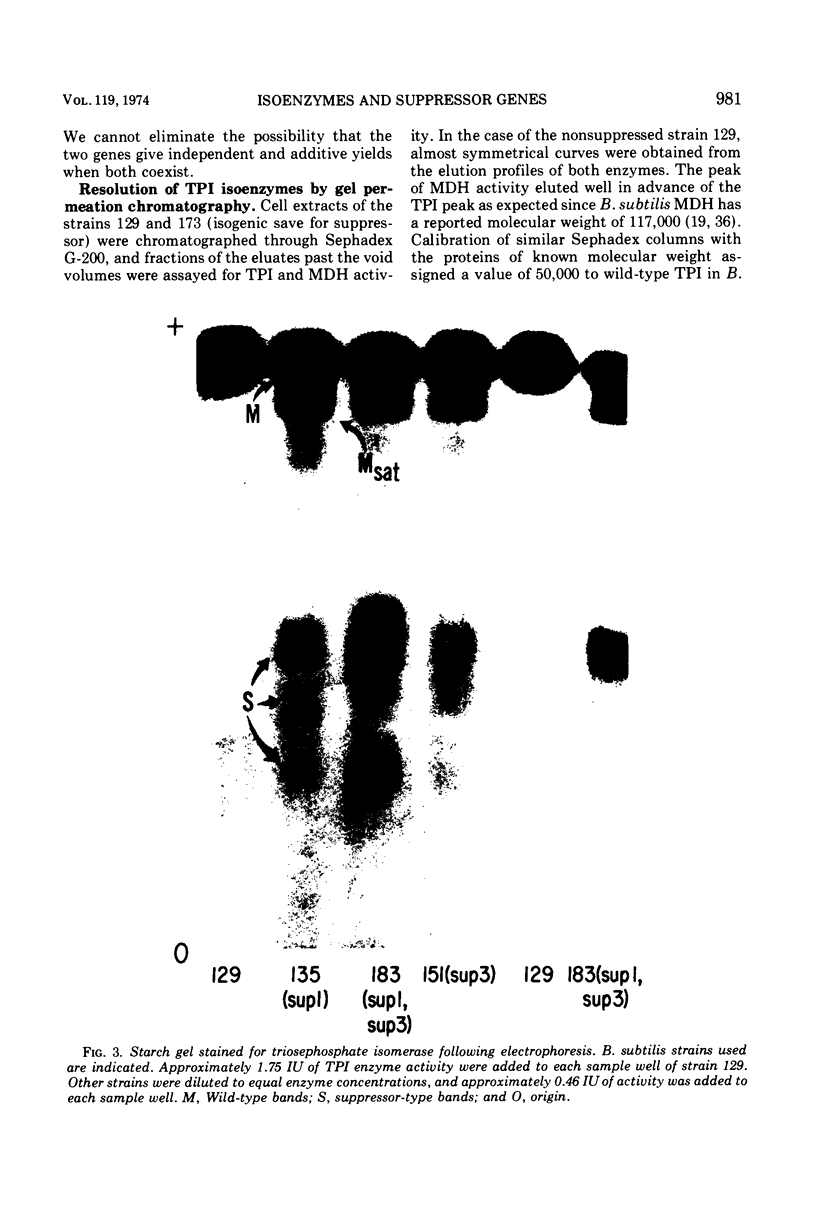
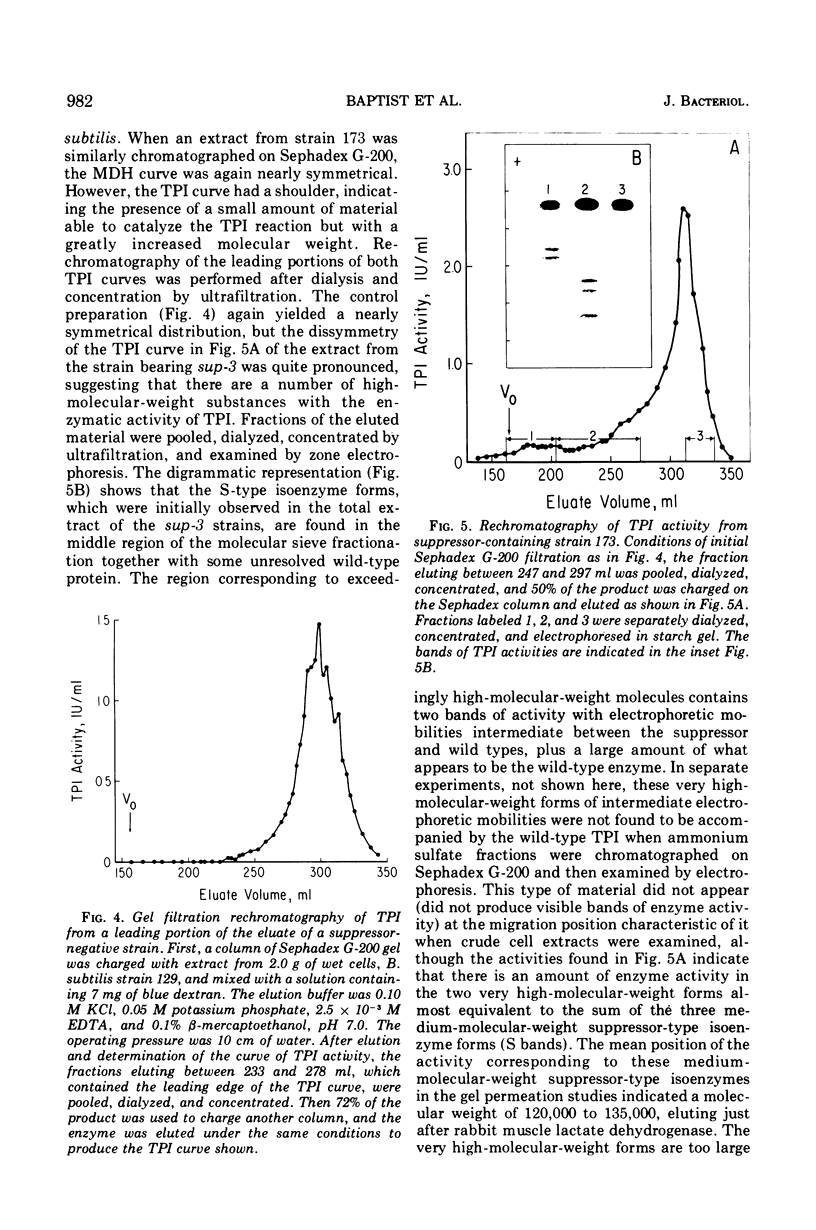
Suppressor mutations in Bacillus subtilis cause the synthesis of a new protein with the enzymatic activity of l-leucine dehydrogenase and two groups of new proteins with the activity of triosephosphate isomerase. The new isoenzymes of triosephosphate isomerase are separable by zone electrophoresis and differ among themselves in elution behavior upon gel permeation chromatography. One group has an apparent average molecular weight of 120,000 to 135,000, which is more than twice that of the wild-type enzyme. Another group appears to be even higher in molecular weight. These data are consistent with the working hypothesis that the new isoenzymes are produced by extension of growing polypeptide chains through one or more chain-terminating triplets, although other mechanisms resulting in alteration of shapes, charges, or associations of the enzymes are not excluded.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baptist J. N., Shaw C. R., Mandel M. Comparative zone electrophoresis of enzymes of Pseudomonas solanacearum and Pseudomonas cepacia. J Bacteriol. 1971 Nov;108(2):799–803. doi: 10.1128/jb.108.2.799-803.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baptist J. N., Shaw C. R., Mandel M. Zone electrophoresis of enzymes in bacterial taxonomy. J Bacteriol. 1969 Jul;99(1):180–188. doi: 10.1128/jb.99.1.180-188.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Bronson M. J., Squires C., Yanofsky C. Nucleotide sequences from tryptophan messenger RNA of Escherichia coli: the sequence corresponding to the amino-terminal region of the first polypeptide specified by the operon. Proc Natl Acad Sci U S A. 1973 Aug;70(8):2335–2339. doi: 10.1073/pnas.70.8.2335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Burton P. M., Waley S. G. The active centre of triose phosphate isomerase. Biochem J. 1966 Sep;100(3):702–710. doi: 10.1042/bj1000702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Contreras R., Ysebaert M., Jou W. M., Fiers W. Bacteriophage Ms2 RNA: nucleotide sequence of the end of the a protein gene and the intercistronic region. Nat New Biol. 1973 Jan 24;241(108):99–101. doi: 10.1038/newbio241099a0. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C. P. Suppressor system in Bacillus subtilis 168. J Bacteriol. 1969 Mar;97(3):1397–1402. doi: 10.1128/jb.97.3.1397-1402.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt R. In vivo degradation of nonsense fragments in E. coli. Nature. 1970 Dec 19;228(5277):1151–1154. doi: 10.1038/2281151a0. [DOI] [PubMed] [Google Scholar]
- Hermier J., Siegenthaler P. A., Blondel-Quéroix J., Bergère J. L. Séparation et propriétés de la leucine déshydrogénase et de l'alanine déshydrogénase de Bacillus subtilis. Bull Soc Chim Biol (Paris) 1965;47(6):1217–1234. [PubMed] [Google Scholar]
- Isono K., Yourno J. Mutation leading to gene fusion in the histidine operon of Salmonella typhimurium. J Mol Biol. 1973 Jun 5;76(4):455–461. doi: 10.1016/0022-2836(73)90484-1. [DOI] [PubMed] [Google Scholar]
- KAPLAN N. O. LACTATE DEHYDROGENASE--STRUCTURE AND FUNCTION. Brookhaven Symp Biol. 1964 Dec;17:131–153. [PubMed] [Google Scholar]
- Kono T., Yourno J. Proteolytic release of a histidinol dehydrogenase fragment from the double enzyme histidinol dehydrogenase-imidazolylacetol-phosphate: L-glutamate aminotransferase. J Biol Chem. 1971 Apr 10;246(7):2203–2206. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARGOLIASH E., SMITH E. L., KREIL G., TUPPY H. Amino-acid sequence of horse heart cytochrome c. Nature. 1961 Dec 23;192:1125–1127. doi: 10.1038/1921125a0. [DOI] [PubMed] [Google Scholar]
- Miller J. C., Waley S. G. Amino acid sequences around the cysteine residues of rabbit muscle triose phosphate isomerase. Biochem J. 1971 Apr;122(2):209–218. doi: 10.1042/bj1220209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min Jou W., Haegeman G., Ysebaert M., Fiers W. Nucleotide sequence of the gene coding for the bacteriophage MS2 coat protein. Nature. 1972 May 12;237(5350):82–88. doi: 10.1038/237082a0. [DOI] [PubMed] [Google Scholar]
- Murphey W. H., Barnaby C., Lin F. J., Kaplan N. O. Malate dehydrogenases. II. Purification and properties of Bacillus subtilis, Bacillus stearothermophilus, and Escherichia coli malate dehydrogenases. J Biol Chem. 1967 Apr 10;242(7):1548–1559. [PubMed] [Google Scholar]
- Nichols J. L. Nucleotide sequence from the polypeptide chain termination region of the coat protein cistron in bacteriophage R17 RNA. Nature. 1970 Jan 10;225(5228):147–151. doi: 10.1038/225147a0. [DOI] [PubMed] [Google Scholar]
- Nichols J. L., Robertson H. D. Sequences of RNA fragments from the bacteriophage f2 coat protein cistron which differ from their R17 counterparts. Biochim Biophys Acta. 1971 Feb 11;228(3):676–681. doi: 10.1016/0005-2787(71)90731-3. [DOI] [PubMed] [Google Scholar]
- Norton I. L., Pfuderer P., Stringer C. D., Hartman F. C. Isolation and characterization of rabbit muscle triose phosphate isomerase. Biochemistry. 1970 Dec 8;9(25):4952–4958. doi: 10.1021/bi00827a019. [DOI] [PubMed] [Google Scholar]
- Okubo S., Yanagida T. Isolation of a suppressor mutant in Bacillus subtilis. J Bacteriol. 1968 Mar;95(3):1187–1188. doi: 10.1128/jb.95.3.1187-1188.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechler M. M., Bruni C. B. Properties of a fused protein formed by genetic manipulation. Histidinol dehydrogenase-imidazolylacetol phosphate: L-glutamate aminotransferase. J Biol Chem. 1971 Mar 25;246(6):1806–1813. [PubMed] [Google Scholar]
- Rechler M. M., Martin R. G. The intercistronic divide: translation of an intercistronic region in the histidine operon of Salmonella typhimurium. Nature. 1970 Jun 6;226(5249):908–911. doi: 10.1038/226908a0. [DOI] [PubMed] [Google Scholar]
- SCOPES R. K. DETECTION OF TRIOSEPHOSPHATE ISOMERASE AFTER ELECTROPHORESIS. Nature. 1964 Feb 29;201:924–925. doi: 10.1038/201924b0. [DOI] [PubMed] [Google Scholar]
- Seideler R. J., Mandel M., Baptist J. N. Molecular heterogeneity of the Bdellovibrios: evidence of two new species. J Bacteriol. 1972 Jan;109(1):209–217. doi: 10.1128/jb.109.1.209-217.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw C. R., Prasad R. Starch gel electrophoresis of enzymes--a compilation of recipes. Biochem Genet. 1970 Apr;4(2):297–320. doi: 10.1007/BF00485780. [DOI] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steitz J. A. Oligonucleotide sequence of replicase initiation site in Q RNA. Nat New Biol. 1972 Mar 22;236(64):71–75. doi: 10.1038/newbio236071a0. [DOI] [PubMed] [Google Scholar]
- THORNE C. J., KAPLAN N. O. Physicochemical properties of pig and horse heart mitochondrial malate dehydrogenase. J Biol Chem. 1963 May;238:1861–1868. [PubMed] [Google Scholar]
- Tevethia M. J., Baptist J. N., Mandel M. Pleiotropic effects of suppressor mutations in Bacillus subtilis. J Bacteriol. 1974 Sep;119(3):961–975. doi: 10.1128/jb.119.3.961-975.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiner A. M., Weber K. A single UGA codon functions as a natural termination signal in the coliphage q beta coat protein cistron. J Mol Biol. 1973 Nov 15;80(4):837–855. doi: 10.1016/0022-2836(73)90213-1. [DOI] [PubMed] [Google Scholar]
- YOSHIDA A. PURIFICATION AND CHEMICAL CHARACTERIZATION OF MALATE DEHYDROGENASE OF BACILLUS SUBTILIS. J Biol Chem. 1965 Mar;240:1113–1117. [PubMed] [Google Scholar]
- Yourno J., Kohno T., Roth J. R. Enzyme evolution: generation of a bifunctional enzyme by fusion of adjacent genes. Nature. 1970 Nov 28;228(5274):820–824. doi: 10.1038/228820a0. [DOI] [PubMed] [Google Scholar]