Abstract
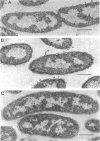
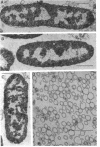
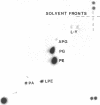
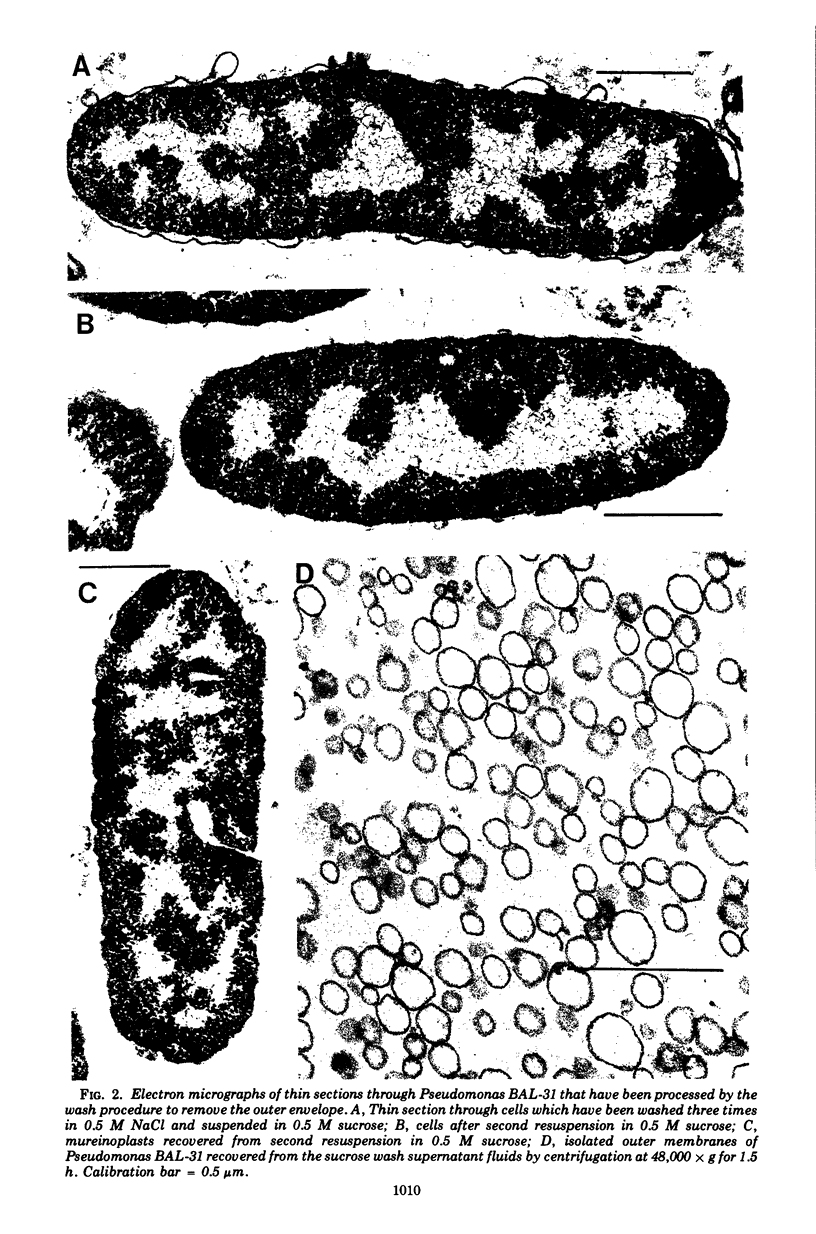
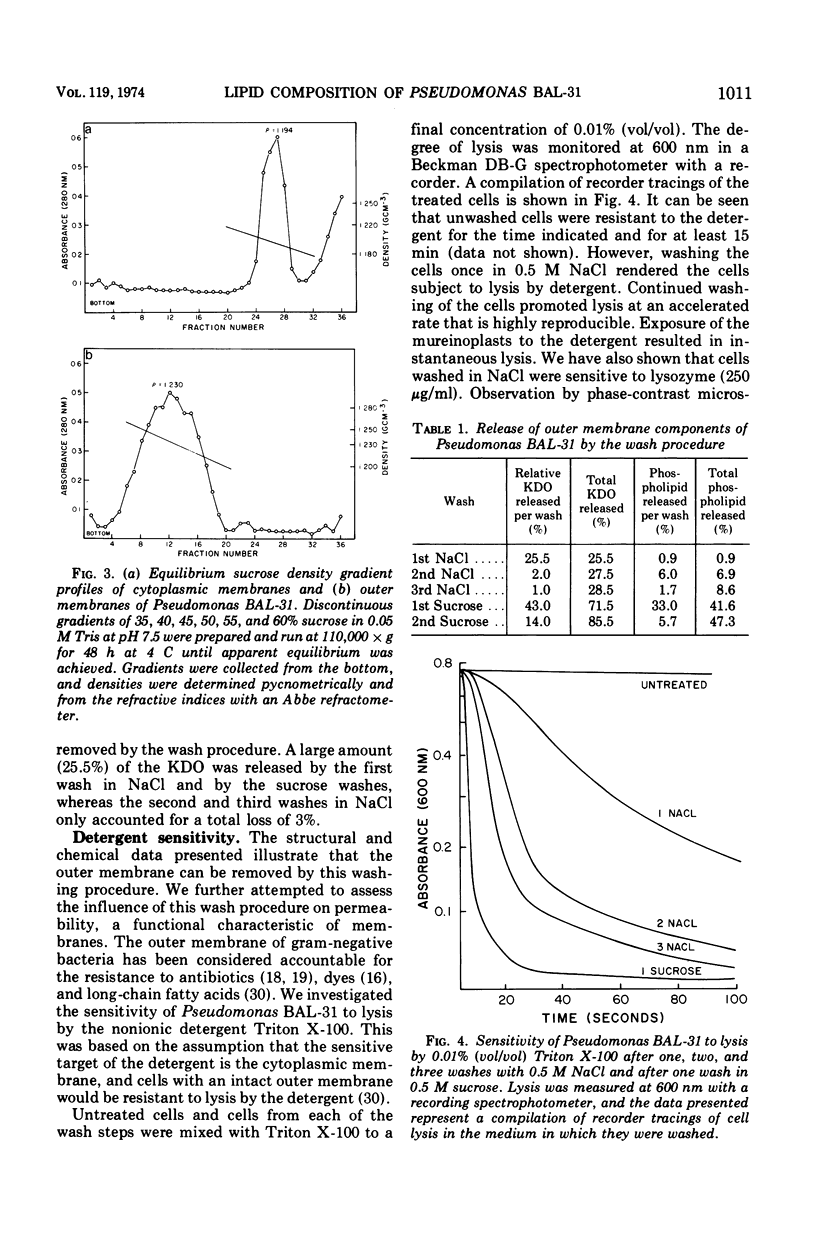
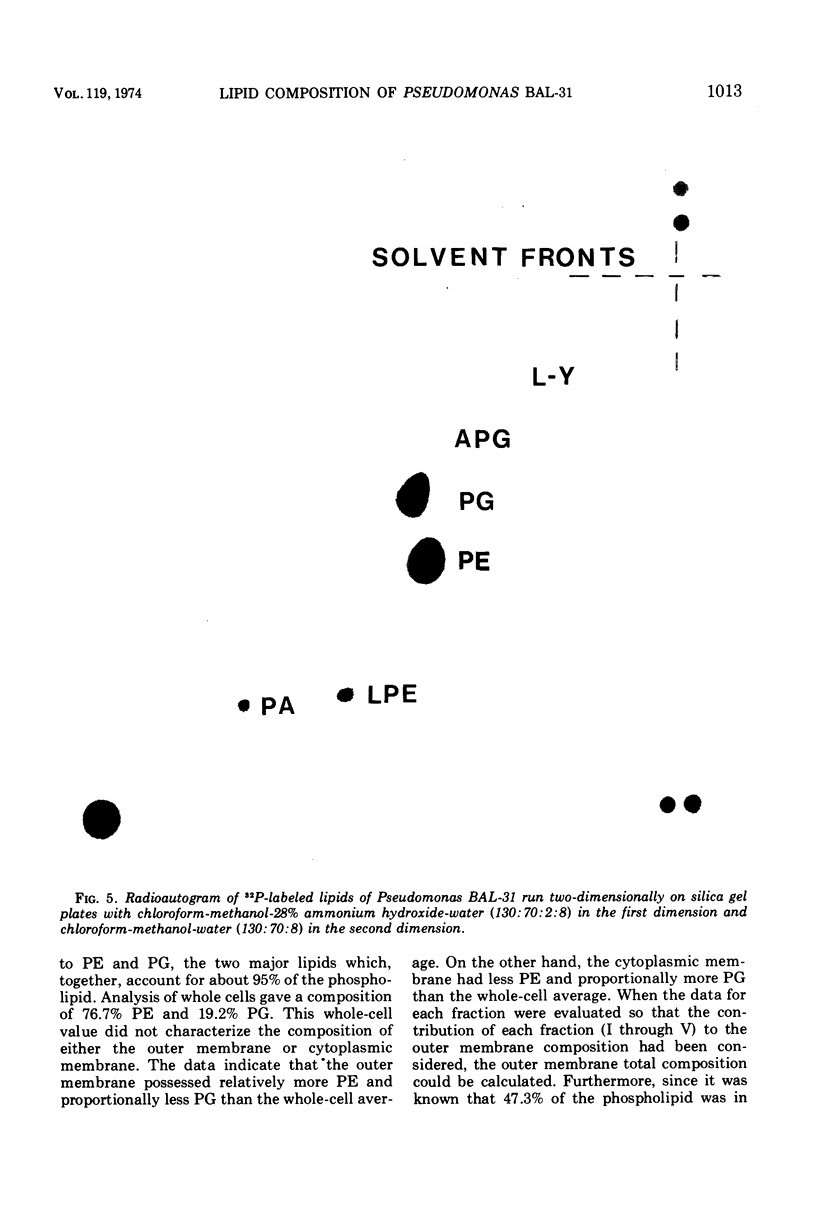
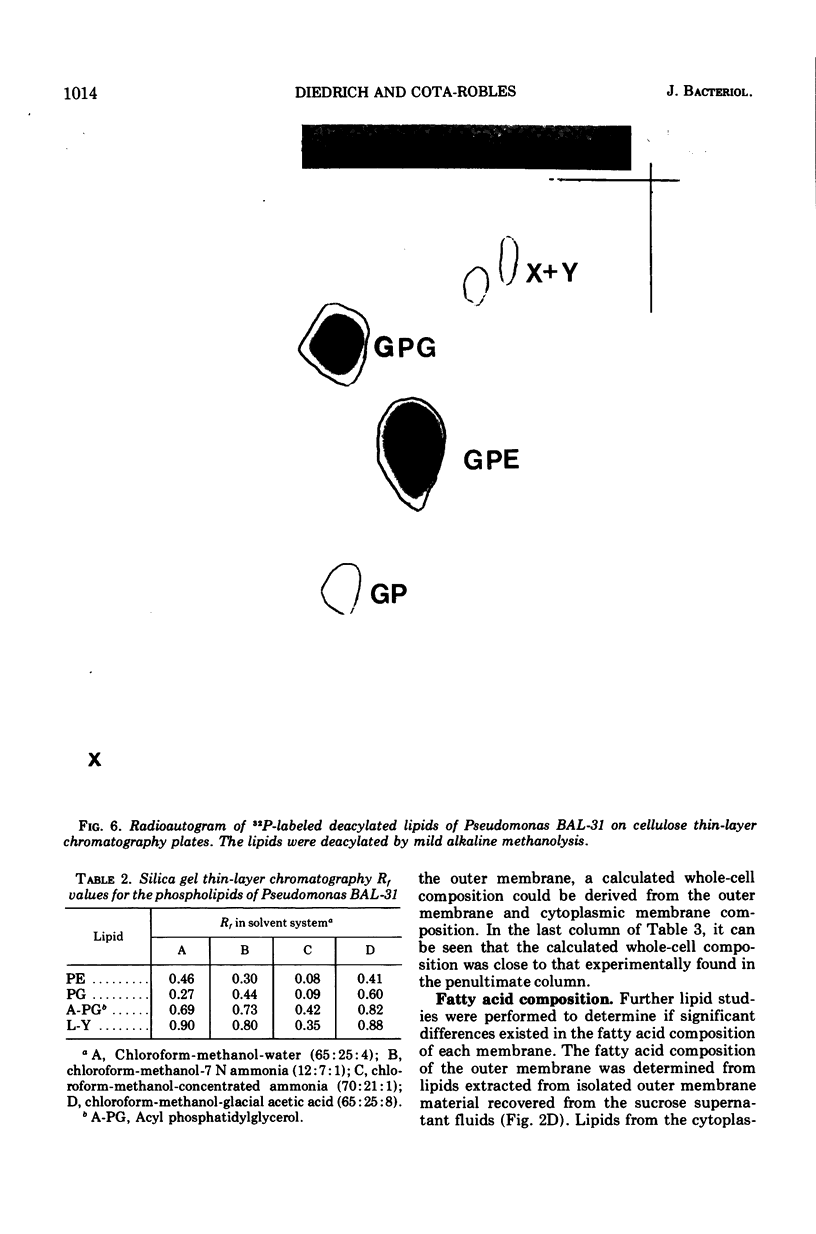
The outer membranes and cytoplasmic membranes of the marine bacterium Pseudomonas BAL-31 were separated by washing the cells three times in 0.5 M NaCl and twice in 0.5 M sucrose. Electron microscopy during the removal of membranes revealed that the outer membranes fragmented in a regular manner to give rise to fairly uniform vesicles measuring approximately 140 nm in diameter. Isolated outer membranes had a buoyant density in sucrose of 1.230 g per cm3, whereas the cytoplasmic membranes had a density of 1.194 g per cm3. The removal of the outer membrane during the application of this procedure was monitored by measuring the release of 2-keto-3-deoxyoctulosonic acid and phospholipid. The cells lost 85.5% of their 2-keto-3-deoxyoctulosonic acid and 47.3% of their phospholipid during this treatment. Complete recovery of outer membrane material could be achieved. The removal of 25.5% of the 2-keto-3-deoxyoctulosonic acid and 0.9% of the phospholipid rendered the cells sensitive to lysis with Triton X-100. The phospholipid composition of the outer membrane was calculated to be 78.9% phosphatidylethanolamine and 16.1% phosphatidylglycerol. The phospholipid composition of the cytoplasmic membrane proved to be 71.5% phosphatidylethanolamine and 23.5% phosphatidylglycerol. The fatty acid composition was also found to be quantitatively heterogeneous between the two membranes.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BLIGH E. G., DYER W. J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959 Aug;37(8):911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- Bell R. M., Mavis R. D., Osborn M. J., Vagelos P. R. Enzymes of phospholipid metabolism: localization in the cytoplasmic and outer membrane of the cell envelope of Escherichia coli and Salmonella typhimurium. Biochim Biophys Acta. 1971 Dec 3;249(2):628–635. doi: 10.1016/0005-2736(71)90144-1. [DOI] [PubMed] [Google Scholar]
- Braunstein S. N., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. V. Phospholipids of the host BAL-31 and of the bacteriophage PM2. Virology. 1971 Mar;43(3):685–695. doi: 10.1016/0042-6822(71)90292-3. [DOI] [PubMed] [Google Scholar]
- Camerini-Otero R. D., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. XII. The fatty acids and lipid content of bacteriophage PM2. Virology. 1972 Aug;49(2):385–393. doi: 10.1016/0042-6822(72)90491-6. [DOI] [PubMed] [Google Scholar]
- Costerton J. W., Forsberg C., Matula T. I., Buckmire F. L., MacLeod R. A. Nutrition and metabolism of marine bacteria. XVI. Formation of protoplasts, spheroplasts, and related forms from a gram-negative marine bacterium. J Bacteriol. 1967 Nov;94(5):1764–1777. doi: 10.1128/jb.94.5.1764-1777.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cota-Robles E., Espejo R. T., Haywood P. W. Ultrastructure of bacterial cells infected with bacteriophage PM2, a lipid-containing bacterial virus. J Virol. 1968 Jan;2(1):56–68. doi: 10.1128/jvi.2.1.56-68.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DITTMER J. C., LESTER R. L. A SIMPLE, SPECIFIC SPRAY FOR THE DETECTION OF PHOSPHOLIPIDS ON THIN-LAYER CHROMATOGRAMS. J Lipid Res. 1964 Jan;5:126–127. [PubMed] [Google Scholar]
- Dahlberg J. E., Franklin R. M. Structure and synthesis of a lipid-containing bacteriophage. IV. Electron microscopic studies of PM2-infected Pseudomonas BAL-31. Virology. 1970 Dec;42(4):1073–1086. doi: 10.1016/0042-6822(70)90355-7. [DOI] [PubMed] [Google Scholar]
- De Petris S. Ultrastructure of the cell wall of Escherichia coli and chemical nature of its constituent layers. J Ultrastruct Res. 1967 Jul;19(1):45–83. doi: 10.1016/s0022-5320(67)80059-5. [DOI] [PubMed] [Google Scholar]
- De Voe I. W., Thompson J., Costerton J. W., MacLeod R. A. Stability and comparative transport capacity of cells, mureinoplasts, and true protoplasts of a gram-negative bacterium. J Bacteriol. 1970 Mar;101(3):1014–1026. doi: 10.1128/jb.101.3.1014-1026.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Voe I. W., Thompson J., Costerton J. W., MacLeod R. A. Stability and comparative transport capacity of cells, mureinoplasts, and true protoplasts of a gram-negative bacterium. J Bacteriol. 1970 Mar;101(3):1014–1026. doi: 10.1128/jb.101.3.1014-1026.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DePamphilis M. L., Adler J. Attachment of flagellar basal bodies to the cell envelope: specific attachment to the outer, lipopolysaccharide membrane and the cyoplasmic membrane. J Bacteriol. 1971 Jan;105(1):396–407. doi: 10.1128/jb.105.1.396-407.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties and characterization of the host bacterium of bacteriophage PM2. J Bacteriol. 1968 May;95(5):1887–1891. doi: 10.1128/jb.95.5.1887-1891.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson P., Nordström K., Normark S. Outer penetration barrier of Escherichia coli K-12: kinetics of the uptake of gentian violet by wild type and envelope mutants. J Bacteriol. 1973 Nov;116(2):893–900. doi: 10.1128/jb.116.2.893-900.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A. Cell wall and cytoplasmic membrane of Escherichia coli. J Biophys Biochem Cytol. 1958 May 25;4(3):323–326. doi: 10.1083/jcb.4.3.323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LEIVE L. A NONSPECIFIC INCREASE IN PERMEABILITY IN ESCHERICHIA COLI PRODUCED BY EDTA. Proc Natl Acad Sci U S A. 1965 Apr;53:745–750. doi: 10.1073/pnas.53.4.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leive L. Studies on the permeability change produced in coliform bacteria by ethylenediaminetetraacetate. J Biol Chem. 1968 May 10;243(9):2373–2380. [PubMed] [Google Scholar]
- OSBORN M. J. STUDIES ON THE GRAM-NEGATIVE CELL WALL. I. EVIDENCE FOR THE ROLE OF 2-KETO- 3-DEOXYOCTONATE IN THE LIPOPOLYSACCHARIDE OF SALMONELLA TYPHIMURIUM. Proc Natl Acad Sci U S A. 1963 Sep;50:499–506. doi: 10.1073/pnas.50.3.499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen R. W., Ballou C. E. Acyl phosphatidylglycerol. A new phospholipid from Salmonella typhimurium. J Biol Chem. 1971 May 25;246(10):3305–3313. [PubMed] [Google Scholar]
- Ono Y., White D. C. Cardiolipin-specific phospholipase D activity in Haemophilus parainfluenzae. J Bacteriol. 1970 Jul;103(1):111–115. doi: 10.1128/jb.103.1.111-115.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osborn M. J., Gander J. E., Parisi E., Carson J. Mechanism of assembly of the outer membrane of Salmonella typhimurium. Isolation and characterization of cytoplasmic and outer membrane. J Biol Chem. 1972 Jun 25;247(12):3962–3972. [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- REPASKE R. Lysis of gram-negative organisms and the role of versene. Biochim Biophys Acta. 1958 Nov;30(2):225–232. doi: 10.1016/0006-3002(58)90044-1. [DOI] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. H., White D. C., Brock T. D. Effect of growth temperature on the lipid composition of Thermus aquaticus. J Bacteriol. 1971 Oct;108(1):227–235. doi: 10.1128/jb.108.1.227-235.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Effect of ethylenediaminetetraacetic acid, Triton X-100, and lysozyme on the morphology and chemical composition of isolate cell walls of Escherichia coli. J Bacteriol. 1971 Oct;108(1):553–563. doi: 10.1128/jb.108.1.553-563.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnaitman C. A. Protein composition of the cell wall and cytoplasmic membrane of Escherichia coli. J Bacteriol. 1970 Nov;104(2):890–901. doi: 10.1128/jb.104.2.890-901.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheu C. W., Freese E. Lipopolysaccharide layer protection of gram-negative bacteria against inhibition by long-chain fatty acids. J Bacteriol. 1973 Sep;115(3):869–875. doi: 10.1128/jb.115.3.869-875.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short S. A., White D. C., Aleem M. I. Phospholipid metabolism in Ferrobacillus ferrooxidans. J Bacteriol. 1969 Jul;99(1):142–150. doi: 10.1128/jb.99.1.142-150.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spurr A. R. A low-viscosity epoxy resin embedding medium for electron microscopy. J Ultrastruct Res. 1969 Jan;26(1):31–43. doi: 10.1016/s0022-5320(69)90033-1. [DOI] [PubMed] [Google Scholar]
- Tucker A. N., White D. C. Heterogeneity of phospholipid composition in the bacterial membrane. J Bacteriol. 1970 May;102(2):508–513. doi: 10.1128/jb.102.2.508-513.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. A., Albright F. R., Lennarz W. J., Schnaitman C. A. Distribution of phospholipid-synthesizing enzymes in the wall and membrane subfractions of the envelope of Escherichia coli. Biochim Biophys Acta. 1971 Dec 3;249(2):636–642. doi: 10.1016/0005-2736(71)90145-3. [DOI] [PubMed] [Google Scholar]
- White D. C., Cox R. H. Indentification and localization of the fatty acids in Haemophilus parainfluenzae. J Bacteriol. 1967 Mar;93(3):1079–1088. doi: 10.1128/jb.93.3.1079-1088.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White D. C. Lipid composition of the electron transport membrane of Haemophilus parainfluenzae. J Bacteriol. 1968 Oct;96(4):1159–1170. doi: 10.1128/jb.96.4.1159-1170.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilkinson B. J., Morman M. R., White D. C. Phospholipid composition and metabolism of Micrococcus denitrificans. J Bacteriol. 1972 Dec;112(3):1288–1294. doi: 10.1128/jb.112.3.1288-1294.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]