Abstract
Long-term potentiation (LTP) in the hippocampal slice preparation has been proposed as an in vitro model for long-term memory. However, correlation of LTP with memory in living animals has been difficult to demonstrate. Furthermore, in the last few years evidence has accumulated that dissociate the two. Because potassium channels might determine the weight of synapses in networks, we studied the role of Kv1.4, a presynaptic A-type voltage-dependent K+ channel, in both memory and LTP. Reverse transcription–PCR and Western blot analysis with specific antibodies showed that antisense oligodeoxyribonucleotide to Kv1.4 microinjected intraventricularly into rat brains obstructed hippocampal Kv1.4 mRNA, “knocking down” the protein in the hippocampus. This antisense knockdown had no effect on rat spatial maze learning, memory, or exploratory behavior, but eliminated both early- and late-phase LTP and reduced paired-pulse facilitation (a presynaptic effect) in CA1 pyramidal neurons without affecting dentate gyrus LTP. This presynaptic Kv1.4 knockdown together with previous postsynaptic Kv1.1 knockdown demonstrates that CA1 LTP is neither necessary nor sufficient for rat spatial memory.
Changes in synaptic efficacy are thought to play a key role in the formation of long-lasting memories. Because long-term potentiation (LTP) represents such a change, it has been proposed as a cellular mechanism that underlies memory (1, 2). However, correlation of LTP with memory in living animals has been difficult to demonstrate. LTP in the hippocampus, a brain area that appears to be necessary for spatial memory (3), requires induction with nonphysiological stimulus parameters (1). Although LTP-related phenomena co-occur with memory in some experimental models (4, 5), there are several observations that have begun to separate the two (6–9). Here, we report that CA1 LTP and spatial memory are differentially affected by long-term changes in the expression of a specific type of potassium channel, Kv1.4. These differences, together with a previous antisense “knockdown” of Kv1.1 (10), establish a clear dissociation between LTP and spatial memory.
In the mammalian central nervous system, more than 40 different potassium channels have been identified. These channels differ in their electrophysiological, neuroanatomical, and cellular distribution (11–13). Memory mutants (primarily the shaker mutants) strongly suggest a role for specific potassium channels in learning and memory (14). Rabbit nictitating membrane conditioning is correlated with enhanced postsynaptic responses caused by persistent reduction of voltage-dependent K+ current in hippocampal cells. Hermissenda voltage-dependent K+ currents are reduced and inactivated more rapidly during and after associative training (15, 16). Furthermore, antisense inhibition of a late rectifying potassium channel, Kv1.1, has been found to disrupt spatial memory without affecting LTP (9).
The diversity and complexity of potassium channels, together with previous findings correlating memory and K+ channels, make them promising candidates for fine-tuning the weights of synapses in networks and thus for characterizing voltage-dependent receptor properties that might affect synaptic mechanisms such as LTP. However, potassium channels are highly conserved throughout evolution, and their pore areas show great similarity among diverse potassium channels (17, 18). Thus, it is very difficult to separate the physiological effects of the channels by using traditional pharmacological approaches. Drosophila memory mutant and mouse transgenic manipulations have been used to study the physiological role(s) of potassium channels. These manipulations, however, may alter the development of brain networks as well as network function, complicating the interpretation of the results. To overcome these obstacles, we took a different approach: knocking down specific potassium channels through intraventricular microinjection of specific antisense oligodeoxyribonucleotide (ODN) in adult animals. This approach has been improved and more successfully implemented in the last few years (19–21).
METHODS
Design of Antisense ODN.
ODN was designed according to the published sequence (11), 20 bases in length, phosphorotioate-protected by a 3′ and 5′ end double substitution, synthesized on a 10 μmol scale and purified on an HPLC (Genosys, The Woodlands, TX). The ODNs were incubated at 37°C for 10 min, in the presence of the cationic lipid DOTAP (N-[1-(2,3-dioleoyloxy)propyl]-N,N,N-trimethylammonium methylsulfate) (13 μM) (Boehringer-Mannheim). The sequence of the antisense ODN to KV1.4 was 5′-GCCACCTCCATGGTGGTAGT-3′. The control was the sense sequence: 5′-ACTACCACCATGGAGGTGGC-3′ (residues 483–502).
Rats and Surgery.
Male Wistar rats from Charles River Breeding Laboratories were used (200–250 g). All experiments were carried out according to the National Institutes of Health animal care guideline. Rats were surgically equipped with two stainless steel guide cannulas 0.5 mm above each lateral ventricle by using a stereotactic apparatus (Kopf Instruments, Tujunga, CA). Coordinates according to G. Paxinos and C. Watson (40) were: anterior-posterior = 0.5 mm, lateral = 1.5 mm, horizontal = 3.2 mm. Rats were allowed a recovery period of 7 days before the first ODN injection. ODNs were injected every 24 hr for 4 days before the first training day (see Fig. 1B). Injections were continued during training. Training was carried out in the morning, and the rats were injected in the afternoon.
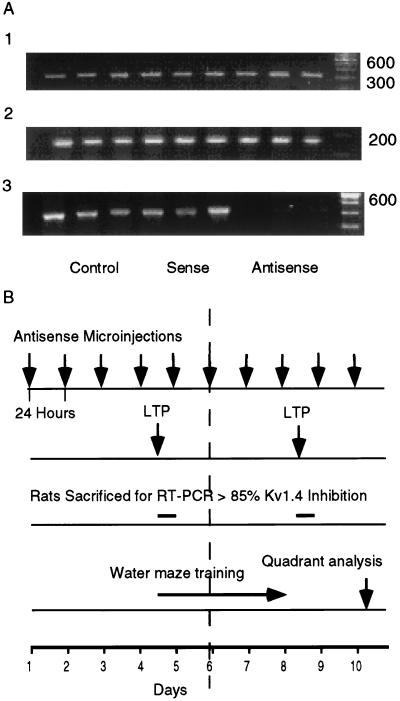
Figure 1.
(A) ODN analysis of Kv1.4 mRNA by RT-PCR. mRNA amplification from hippocampi of nine rats injected for 4 days with either sham, sense, or antisense to Kv1.4. (A1) Kv1.1; (A2) PGK1; (A3) Kv1.4. (B) Time chart of antisense microinjection performed every 24 hr as indicated by arrows on top line. Time chart for LTP (second line) measured after either 4 or 8 days of microinjection. Rats sacrificed for RT-PCR and Western blots after 4 or 8 days (third line). Water maze training on each of 4 consecutive days between the fourth and eighth days (fourth line). Quadrant analysis was performed on the 10th day, 2 days after the last training day.
Reverse Transcription–PCR (RT-PCR) and Western Blot Analysis of Hippocampus from Injected Rats.
Rats were sacrificed 24 hr after the last of either 4 or 8 days of antisense injections. The brains were removed, and the hippocampi were immediately frozen on dry ice. RNA was extracted by an RNA Isolator (Genosys). RNA yield was 100 ± 25 μg/hippocampus. Forward (F) and reverse (R) primers used to identify Kv1.4, Kv1.1, and phosphoglycerate kinase 1 (PGK1) were based on the reported sequence (11, 17, 39). Oligonucleotide sequences and location of the primers were: PGK1, F-5′-AGGTGCTCAACAACATGGAG-3′ (residues 777–796), R-5′-TACCAGAGGCCACAGTAGCT-3′ (residues 940–959); Kv1.1 F-5′-GTAGACCTCTGAACCTTCTGG-3′ (residues 86–106), R-5′-AGAGTCTTGAGCTGCGTCTCC-3′ (residues 392–411); Kv1.4, F-5′-AGCAATGCTGAGTCTCTGAG-3′ (residues 284–303), R-5′-GTGTCTGATGATGATGGTGG-3′ (residues 674–693). RNA was reverse-transcribed by using Moloney murine leukemia virus-RT (GIBCO/BRL). PCR was performed on a Perkin–Elmer cetus thermal cycler by using Taq DNA polymerase, Taq buffer containing 2.5 mM MgCl2 (Perkin–Elmer): for PGK1, 27 cycles of 94°C for 1 min, 59°C for 1 min, and 72°C for 1 min; for Kv1.1, 35 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min; and for Kv1.4, 35 cycles of 94°C for 1 min, 58°C for 1 min, and 72°C for 1 min. Amplification products arising from RT-PCR were electrophoresed on a 2% agarose gel and visualized by ethidium bromide staining.
For Western blot analysis each hippocampus was homogenized in 2× lysis buffer (2.3% SDS/5% β-mercaptoethanol/10% glycerol/0.025 M Tris, pH 6.8) and boiled for 5 min. Protein (100 μg/lane) was loaded on a SDS/PAGE (gradient 4–20%). The antibodies used were polyclonal antiKv1.4 (Alomone Labs, Jerusalem, Israel), and polyclonal anti-NMDAR1 (Chemicon) with subsequent staining with horseradish peroxidase-Protein A and detected by ECL (Pierce).
Rat Behavior Paradigms.
Water maze training procedure was according to Morris et al. (22) and modified as described by Meiri et al. (10). The rats were given three training trails each day for 4 consecutive days, each trial lasting up to 120 sec. Two days after the end of training the island was removed and the search strategy of the rats was monitored (quadrant analysis). Open-field exploratory behavior was monitored in the same pool as the water maze but without water, a day after quadrant analysis. Distance of swimming was monitored as the rats’ first encounter with the pool.
Hippocampal Slices Preparation and CA1 Electophysiological Recordings.
Brains were removed and cooled rapidly in modified 119 mM NaCl, 2.5 mM KCl, 2.5 mM CaCl2, 1.3 mM MgSO4, 1 mM NaH2PO4, 26.2 mM Na2HCO3, and 11 mM glucose (gassed with 95% O2-5% CO2) (ACSF). The slices (400 μm thick) were cut with a McIlwain Tissue Chopper (Mickle Laboratory Engineering, Gomshall, Surrey, U.K.) and kept in an interface chamber in ACSF solution. Neurons were recorded with glass micropipettes (filled with 3 M K acetate, pH 7.25) in the cell body layer of the CA1 region. One stimulation electrode was positioned in the stratum radiatum of the CA1 subfield to stimulate the Schaffer collateral pathway. Test stimulation intensity (30–50 μA, 50 μs, 0.1 Hz) was set to elicit an excitatory postsynaptic potential (EPSP) that was 30–50% amplitude of the threshold for evoking action potentials. The EPSPs were evaluated as their initial slope, measured by using linear regression of the first ms of the linear rising phase of EPSPs. After recording 10 min of baseline responses, two trains of tetanic stimulation (100 Hz, 100 μs for 1 s each at a 2-s interval) were administered to induce LTP. Percent baseline slope of EPSPs was calculated as: slope at each minute taken from the averaged value of three traces divided by the mean baseline slope × 100. Baseline slope was the mean of 10 min before tetanization. For late-phase LTP (L-LTP) induction, three trains of tetanic stimulation (100 Hz, 100 μs for 1 s each at 5-min intervals) were administered.
LTP in the Dentate Gyrus.
Stimulation, with electrode tips situated in perforant path fibers in stratum lacunosum-moleculare, consisted of 50-μs duration monophasic constant current pulses delivered once per min, at stimulus intensities that were 30–50% below the threshold for evoking an action potential. Four sets of tetanic stimulation were administered to induce LTP, separated by intervals of 10 min. Each set contained five trains, 10 pulses (200 μs) per train at 400 Hz, one train per s for 5 s.
RESULTS
Antisense ODN (20 bases in length) was designed complementary to the mRNA AUG sequence region of Kv1.4. The ODN was checked for absence of significant homology with other mammalian sequences and for minimization of potential hairpin and duplex formation (11). Rats were surgically equipped with two cannulae in the lateral ventricle. Direct cannulation of the hippocampus was avoided to prevent lesioning and/or gliosis, which is caused when a cannula is left in the hippocampus for long periods of time. Intraventricular microinjection of the antisense ODN caused a sustained marked reduction (by 85 ± 4%) of hippocampal Kv1.4 mRNA after four and eight consecutive antisense injections, as measured with RT-PCR. A second “shaker” channel (Kv1.1) and a housekeeping gene (PGK1) were not affected by the antisense treatment, demonstrating the specificity of the inhibition (Fig. 1). As a result of the mRNA inhibition, the amount of the Kv1.4 protein was significantly decreased (by 40 ± 8%) as measured with Western blot analysis (see Fig. 3F), whereas the number of glutamatergic N-methyl-d-aspartic acid-NR1 receptors was not affected by the antisense (see Fig. 3F).
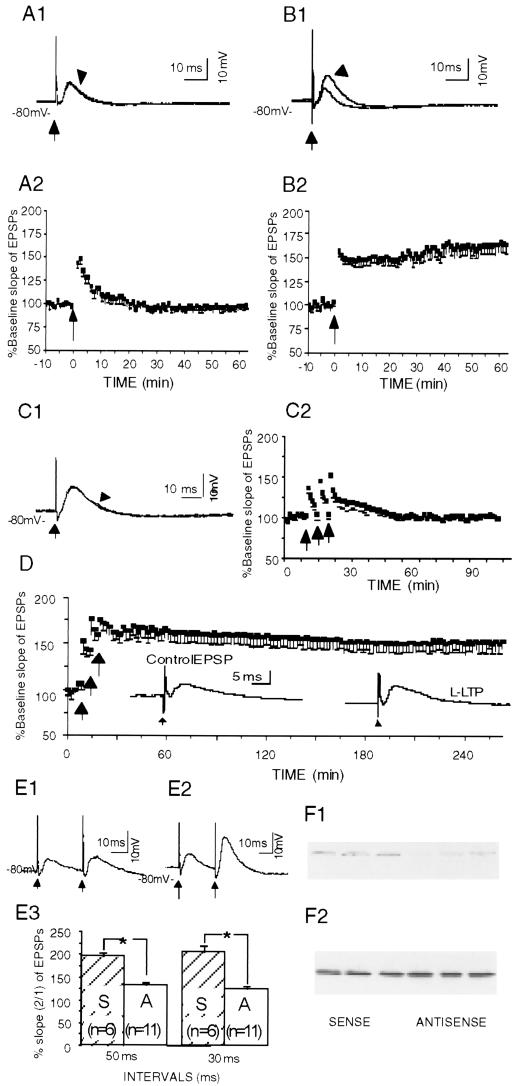
Figure 3.
LTP was eliminated and PPF was reduced in CA1 pyramidal neurons from Kv1.4 antisense-treated animals. (A) Lack of LTP in CA1 pyramidal neurons from the antisense-treated rats, showing an example of EPSPs (A1) evoked with single-pulse stimulation of the Schaffer collateral pathway (at arrow) and plotted group data (A2). Arrowhead in A1 points to a trace obtained 60 min after the tetanization, whereas the other trace was obtained 5 min before the tetanization. In A2, the arrow indicates the time of tetanization. (B) A tetanic train (arrow in B2) induced LTP in CA1 pyramidal neurons from sense-treated rats. The EPSP observed 60 min after tetanization (indicated by the arrowhead in B1) was potentiated as compared with that 5 min before tetanization (the unmarked trace in B1). (C) L-LTP was not induced in the antisense-treated group. Sample trace (indicated by the arrowhead in (C1) obtained with single-pulse stimulation of the Schaffer collateral pathway 70 min after the last train did not differ from the other trace obtained 5 min before the first train of stimulation. The tetanic trains induced a smaller and short-lasting posttetanic potentiation but the potentiation was not maintained (C2). (D) L-LTP was induced in the sense-treated group. For clarity, only every other data point is shown. (Inset) Traces were obtained 5 min before the first train (Control EPSP) and 4 hr after the last train (LTP). (E) PPF was largely inhibited in neurons from antisense-treated rats (E1) as compared with that from sense-treated rats (E2). The difference was significant (E3) at inter-stimulus intervals of either 30 or 50 ms (P < 0.05, unpaired t test). Plotted data are shown as means + SEM. (F) Western blot analysis of homogenate from six rats injected with either sense or antisense (F1) Kv1.4 (F2) N-methyl-d-aspartic acid-NR1.
Rat water maze spatial memory previously has been established as hippocampus dependent. Lesions of the hippocampus or protein synthesis inhibition in the hippocampus results in a long-term impairment in rat water maze performance (22, 23). Further evidence emphasizes the essential role of CA1 neurons of the hippocampus in spatial learning. Bilateral lesions, limited to the CA1 field, are sufficient to produce memory loss in patients and impair rats’ ability to solve spatial tasks (24). The extent of CA1 cell loss correlates with water maze deficits, but can be restored with primary fetal cell suspension transplants containing CA1 pyramidal cells. Transplants containing cholinergic cells from the basal forebrain, granule cells from the dentate gyrus, or a different class of hippocampal pyramidal cells (CA3) are ineffective (25–27). These observations are consistent with the evidence that the dentate gyrus does not seem to be required for water maze performance (6). The importance of the hippocampus in spatial learning is further supported by recordings from place cells, which are believed to be CA1 pyramidal cells that fire selectively in correlation with spatial locations (28).
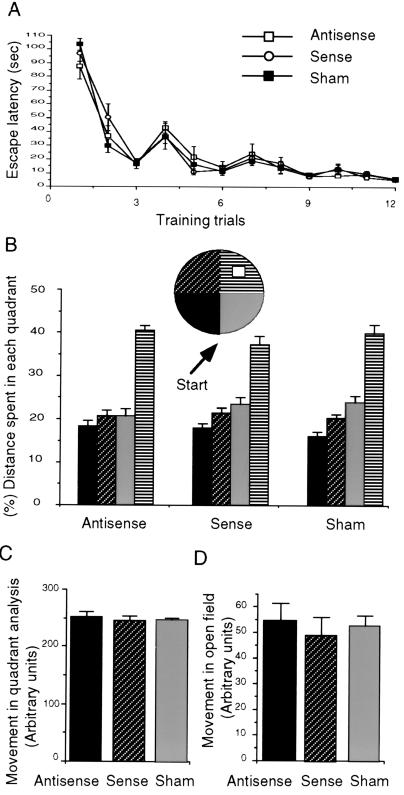
Here we tested the effect of antisense ODN-induced reduction of Kv1.4 on rat performance in the water maze (29). Bilateral injections of the Kv1.4 antisense into the lateral ventricle had no effect on either learning or memory of the location of a hidden platform in the water maze. Latency to find the platform was indistinguishable between antisense, sense, or sham-injected rats (Fig. 2A). ANOVA with repeated measures showed no significant difference caused by administering the Kv1.4 antisense and no interaction between different groups and days of training. The effect of session of training was significant (F11.446 = 24.78, n = 24, P < 0.001), indicating that all groups reduced their escape latency in correlation with the amount of training. Whether the rats used spatial memory to locate the platform was evaluated 48 hr after the last of 4 training days by measuring the rats’ swimming pattern in the pool without the hidden platform (Fig. 2B). All three groups spent more time in the quadrant where the platform had been placed during the training than in each of the other three quadrants (ANOVA antisense, F3.84 = 6.7, n = 10, P < 0.001; sense, F3.66 = 7.3, n = 10, P < 0.002; sham F3.39 = 8.94, n = 10, P < 0.005). Neither exploratory behavior in an open field (antisense 54 ± 7, n = 10; sense 48 ± 8, n = 10; sham 52 ± 4, n = 10 arbitrary units) nor swimming distance in the water pool (antisense 254 ± 9, n = 10; sense 244 ± 5, n = 10; sham 240 ± 4, n = 10, arbitrary units) was affected by the Kv1.4 antisense administration (Fig. 2 C and D).
Figure 2.
Effect of antisense on rat spatial memory. (A) ODNs were injected every 24 hr for 4 days before the first training day. Injections were continued during training every day. Rats were given three training trails each day for 4 consecutive days each trial lasting up to 120 s. Escape latency was measured by using a stopwatch. (B) Two days after training the island was removed, and the search strategy of the rat was monitored to determine whether the rat searched for the island in the quadrant where the island was located previously. (C) Distance of swimming in the first encounter with the pool. (D) Open-field exploratory behavior was monitored 1 day after the quadrant analysis in the same pool as the water maze but without water. All data are shown as means ± SEM.
We then undertook a thorough study of hippocampal LTP, an important cellular model for memory formation. Tetanic trains induced a brief posttetanic potentiation of the EPSPs (Fig. 3A) in the CA1 neurons from Kv1.4 antisense-treated (4 or 8 days) rats. LTP, however, was not induced (Fig. 3A). The % baseline slope of EPSPs 40 min after the tetanization (97.5 ± 3.2%, n = 10, P > 0.05; paired t test) did not differ significantly from the value before the tetanization. In contrast, the tetanic train induced both posttetanic potentiation and LTP in the neurons from control, Kv1.4 sense-treated rats (Fig. 3B). Forty minutes after the tetanization, % baseline slope of EPSPs (165.1 ± 9.5%, n = 8, P < 0.05; paired t test) increased significantly for sense-treated rats. The groups were significantly different from each other (F1, 16 = 17.9; P < 0.001), demonstrating that Kv1.4 antisense treatment disrupted LTP induction. Input resistance (100.6 ± 4.8 MΩ, n = 20, for the neurons from the antisense-treated rats vs. 99.1 ± 3.2 MΩ, n = 23, for the neurons from the sense-treated animals) did not differ between the groups (P > 0.05, unpaired t test).
We further examined the effects of Kv1.4 antisense on L-LTP, a recently defined form of synaptic plasticity that requires de novo protein synthesis (30, 31). A set of three tetanic trains that induced L-LTP in the CA1 pyramidal neurons from the sense-injected rats (Fig. 3D) failed to induce L-LTP in the neurons from Kv1.4 antisense-injected rats (Fig. 3C). Thus, in the antisense-treated group the EPSPs after the tetanization returned rapidly to their baseline slope with an average % baseline EPSP slope of 99.5% (± 3.0%, n = 5, P > 0.05, paired t test) 40 min after the last train, whereas in the sense-treated group, the EPSP potentiation was sustained and had an average % baseline EPSP slope of 164.0% (± 9.4%, n = 4, P < 0.05; paired t test) 40 min after the last tetanic train.
To examine whether the LTP blocking effect was caused by a change in presynaptic neurotransmitter release, we analyzed paired-pulse facilitation (PPF), a phenomenon by which a second synaptic stimulation of equal magnitude at a short interval elicits a larger synaptic response than the first. A short-term increase in presynaptic intracellular calcium levels has been implicated as an underlying mechanism for PPF, which thus has been used to evaluate presynaptic involvement (32, 33). PPF was significantly suppressed in the neurons from the antisense-treated rats, as compared with that from the sense-treated animals (Fig. 3E). These data, coupled with histological evidence of a predominant presynaptic localization of the Kv1.4 channels (34–36), suggest that decreased presynaptic transmitter release contributes to the blockade of the LTP in the antisense-treated animals.
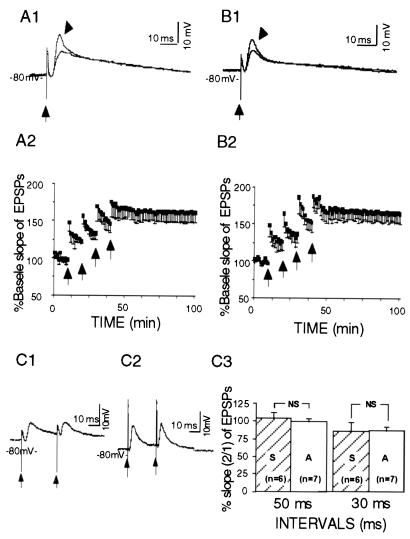
In contrast to CA1 pyramidal cells, granule cells of the dentate gyrus from the antisense-treated (Fig. 4A) as well as sense-treated animals (Fig. 4B) did show normal LTP in response to tetanic stimulation of the perforant path. The % baseline slopes of EPSPs were 162.9 (± 13.2%, n = 4, P < 0.05, paired t test) in the antisense-treated group and 165.3 (±13.6%, n = 4, P < 0.05, paired t test) in sense-treated rats, 40 min after the last train. The groups did not significantly differ from each other (F1,6 = 1.57; P > 0.1), demonstrating that Kv1.4 antisense treatment did not affect dentate LTP induction. Paired-pulse stimulation at brief intervals did not produce PPF in granule neurons. The percentage ratio (2/1) of the EPSP slopes in neurons from antisense-treated rats did not differ from that of sense-treated animals (Fig. 4C).
Figure 4.
LTP was induced in dentate granule neurons from Kv1.4 antisense-treated (A) and sense-treated (B) rats, shown as EPSPs (A1 and B1) evoked with single-pulse stimulation of the perforant pathway (at arrows) and plotted group data (A2 and B2). Arrowheads in A1 and B1 point to the traces obtained 60 min after the last train of stimulation, the other traces (unmarked) were obtained 5 min before the first train. (C) PPF was not obvious in the dentate granule neurons from the antisense-treated (C1) and the sense-treated (C2) rats, when stimulated at interstimulus intervals of 30 or 50 ms. No significant difference was observed (C3) between the two groups (P > 0.05, unpaired t test). Plotted data are shown as means ± SEM.
DISCUSSION
Kv1.4 is a member of the shaker super family and a rapidly inactivating A-type potassium channel. It is neuroanatomically located in large areas of the brain, including the hippocampus and the cortex. In the hippocampus, Kv1.4 is most abundant in the dentate gyrus and CA3 and appears in lower density in CA1, localized predominantly in the axons and their presynaptic terminal branches (11). Its potential role in LTP and/or memory is especially intriguing because its inactivation is controlled by Ca2+/calmodulin-dependent protein kinase (CaMKII) and protein phosphatase 2B, two enzymes that have been correlated with both memory and synaptic strength (37, 38). Our finding provides evidence that Kv1.4 channels play an important role in the synaptic transmission from Schaffer collateral terminals onto CA1 pyramidal neurons. Knockdown of these channels eliminates CA1 LTP. This effect most likely depends on presynaptic dysfunction, because PPF, a presynaptic phenomenon, in CA1 neurons was reduced. The CA1 region, but not dentate gyrus, previously has been shown to be essential for rat spatial maze learning. Normal perforant pathway-dentate granule cells LTP in rats treated with Kv1.4 antisense suggests that different cellular mechanisms are responsible for CA1 and dentate LTP. Another possibility that might explain normal dentate gyrus LTP is that the abundant presence of Kv1.4 channels in this region may require a larger percentage reduction in the expression of the channel before a functional change becomes evident.
Antisense to Kv1.4 did not affect the rat’s water maze performance under the condition described in this article (i.e., 4 consecutive training days, three trials a day). These training conditions are similar to those used previously (10) and less intense then those usually used (22, 29) to correlate LTP and water maze spatial memory. Nevertheless, it is still possible that with even less intense training conditions one might see some residual memory deficit. However, because CA1 LTP is proposed as a major cellular mechanism for spatial memory and is completely eliminated because of Kv1.4 antisense treatment, it is remarkable that with the present training protocol water maze performance was not even slightly affected.
All of the above results taken together indicate that the specific antisense designed to bind mRNA for the potassium Kv1.4 subunit reached its expected intracellular mRNA target, altered the expression of the A-type Kv1.4 channels, and caused elimination of both short- and long-phase CA1 LTP, without affecting dentate gyrus LTP. This elimination of CA1 LTP occurred in the absence of any effect on rat performance in the CA1 hippocampus-dependent water maze learning paradigm. This presynaptic Kv1.4 knockdown shows that CA1 LTP is not necessary for rat spatial maze memory. Previous Kv1.1 knockdown experiments showed that CA1 LTP is not sufficient for rat spatial memory (10). Thus, by using antisense knockdown of potassium channels we have been able to dissociate LTP and spatial memory in the hippocampus.
ABBREVIATIONS
- LTP
long-term potentiation
- ODN
oligodeoxyribonucleotide
- RT-PCR
reverse transcription–PCR
- PGK1
phosphoglycerate kinase 1
- EPSP
excitatory postsynaptic potential
- L-LTP
late-phase LTP
- PPF
paired-pulse facilitation
References
- 1.Bliss T V P, Collingridge G L. Nature (London) 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 2.Malenka R C, Nicoll R A. Trends Neurosci. 1993;16:521–527. doi: 10.1016/0166-2236(93)90197-t. [DOI] [PubMed] [Google Scholar]
- 3.Barnes C A. Trends Neurosci. 1988;11:163–168. doi: 10.1016/0166-2236(88)90143-9. [DOI] [PubMed] [Google Scholar]
- 4.Rogan M T, Staubli U V, LeDoux J E. Nature (London) 1997;390:604–607. doi: 10.1038/37601. [DOI] [PubMed] [Google Scholar]
- 5.Silva A J, Paylor R, Wehner J M, Tonegawa S. Science. 1992;257:206–211. doi: 10.1126/science.1321493. [DOI] [PubMed] [Google Scholar]
- 6.Nosten-Bertrand M, Errington M L, Murphy K P, Tokugawa Y, Barboni E, Kozlova E, Michalovich D, Morris R G, Silver J, Stewart C L, et al. Nature (London) 1996;379:826–829. doi: 10.1038/379826a0. [DOI] [PubMed] [Google Scholar]
- 7.Montkowski A, Holsboer F. NeuroReport. 1997;8:779–782. doi: 10.1097/00001756-199702100-00040. [DOI] [PubMed] [Google Scholar]
- 8.Bannerman D M, Butcher S P, Good M A, Morris R G M. Neurobiol Learning Memory. 1997;68:252–270. doi: 10.1006/nlme.1997.3797. [DOI] [PubMed] [Google Scholar]
- 9.Okabe S, Collin C, Auerbach J M, Meiri N, Bengzon J, Kennedy M B, Segal M, McKay R D. J Neurosci. 1998;18:4177–4188. doi: 10.1523/JNEUROSCI.18-11-04177.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meiri N, Ghelardini C, Tesco G, Galeotti N, Dahl D, Tomsic D, Cavallaro S, Quattrone A, Capaccioli S, Bartolini A, Alkon D L. Proc Natl Acd Sci USA. 1997;94:4430–4434. doi: 10.1073/pnas.94.9.4430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stuhmer W, Ruppersberg J P, Schroter K H, Sakmann B, Stocker M, Giese K P, Perschke A, Baumann A, Pongs O. EMBO J. 1989;8:3235–3244. doi: 10.1002/j.1460-2075.1989.tb08483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jan L Y, Jan Y N. Trends Neurosci. 1990;13:415–419. doi: 10.1016/0166-2236(90)90123-r. [DOI] [PubMed] [Google Scholar]
- 13.Roeper J, Pongs O. Curr Opin Neurobiol. 1996;6:338–341. doi: 10.1016/s0959-4388(96)80117-6. [DOI] [PubMed] [Google Scholar]
- 14.Cowan T M, Siegel R W. J Neurogenet. 1984;1:333–344. doi: 10.3109/01677068409107095. [DOI] [PubMed] [Google Scholar]
- 15.Sanchez-Andres J V, Alkon D L. J Neurophysiol. 1991;65:796–807. doi: 10.1152/jn.1991.65.4.796. [DOI] [PubMed] [Google Scholar]
- 16.Alkon D L, Lederhendler I, Shoukimas J J. Science. 1982;215:693–695. doi: 10.1126/science.7058334. [DOI] [PubMed] [Google Scholar]
- 17.Baumann A, Grupe A, Ackermann A, Pongs O. EMBO J. 1988;7:2457–2463. doi: 10.1002/j.1460-2075.1988.tb03092.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Salkoff L, Jegala T. Neuron. 1995;15:489–492. doi: 10.1016/0896-6273(95)90137-x. [DOI] [PubMed] [Google Scholar]
- 19.Robinson E S J, Nutt D J, Jackson H C, Hudson A L. J Psychopharmacol. 1997;11:259–269. doi: 10.1177/026988119701100310. [DOI] [PubMed] [Google Scholar]
- 20.Lamprecht R, Hazvi S, Dudai Y. J Neurosci. 1997;17:8443–8450. doi: 10.1523/JNEUROSCI.17-21-08443.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Guzowski J F, McGaugh J L. Proc Natl Acad Sci USA. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Morris R G M, Garrud P J, Rawlins N P, O’Keefe J. Nature (London) 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- 23.Meiri N, Rosenblum K. Brain Res. 1998;789:48–55. doi: 10.1016/s0006-8993(97)01528-x. [DOI] [PubMed] [Google Scholar]
- 24.Zola-Morgan S, Squire L R, Amaral D G. J Neurosci. 1986;6:2950–2967. doi: 10.1523/JNEUROSCI.06-10-02950.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hodges H, Sowinski P, Fleming P, Kershaw T R, Sinden J D, Meldrum B S, Gray J A. Neuroscience. 1996;72:959–988. doi: 10.1016/0306-4522(96)00004-8. [DOI] [PubMed] [Google Scholar]
- 26.Netto C A, Hodges H, Sinden J D, Le Peillet E, Kershaw T, Sowinski P, Meldrum B S, Gray J A. Neuroscience. 1993;54:69–92. doi: 10.1016/0306-4522(93)90384-r. [DOI] [PubMed] [Google Scholar]
- 27.Sinden J D, Rashid-Doubell F, Kershaw T R, Nelson A, Chadwick A, Jat P S, Noble M D, Hodges H, Gray J A. Neuroscience. 1997;81:599–608. doi: 10.1016/s0306-4522(97)00330-8. [DOI] [PubMed] [Google Scholar]
- 28.O’Keefe J, Burgess N. Nature (London) 1996;381:425–428. doi: 10.1038/381425a0. [DOI] [PubMed] [Google Scholar]
- 29.Morris R G M. J Neurosci Methods. 1984;11:47–59. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 30.Frey U, Morris R G M. Nature (London) 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 31.Sossin W S. Trends Neurosci. 1996;19:215–218. doi: 10.1016/0166-2236(96)20016-5. [DOI] [PubMed] [Google Scholar]
- 32.Schulz P, Cook E, Johnston D. J Neurosci. 1994;14:5325–5337. doi: 10.1523/JNEUROSCI.14-09-05325.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKernan M G, Shinnick-Gallagher P. Nature (London) 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 34.Cooper E C, Milroy A, Jan Y N, Jan LY, Lowestein D H. J Neurosci. 1998;18:965–974. doi: 10.1523/JNEUROSCI.18-03-00965.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sheng M, Tsaur M-L, Jan Y N, Jan L Y. Neuron. 1992;9:271–284. doi: 10.1016/0896-6273(92)90166-b. [DOI] [PubMed] [Google Scholar]
- 36.Veh R W, Lichtinghagen R, Sewing S, Wunder F, Grumbach I M, Pongs O. Eur J Neurosci. 1995;7:2189–2205. doi: 10.1111/j.1460-9568.1995.tb00641.x. [DOI] [PubMed] [Google Scholar]
- 37.Sakakibara M, DeLorenzo R, Goldenring J R, Neary J T, Heldman E, Alkon D L. Biophys J. 1986;50:319–327. doi: 10.1016/S0006-3495(86)83465-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Roeper J, Lorra J C, Pongs O. J Neurosci. 1997;17:3379–3391. doi: 10.1523/JNEUROSCI.17-10-03379.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ciccarese S, Tommasi S, Vonghia G. Biochem Biophys Res Commun. 1989;165:1337–1344. doi: 10.1016/0006-291x(89)92750-2. [DOI] [PubMed] [Google Scholar]
- 40.Paxinos G, Watson C. The Rat Brain in Sterotaxic Coordinates. 2nd Ed. San Diego: Academic; 1982. [Google Scholar]