Abstract
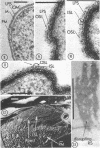
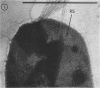
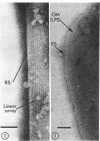
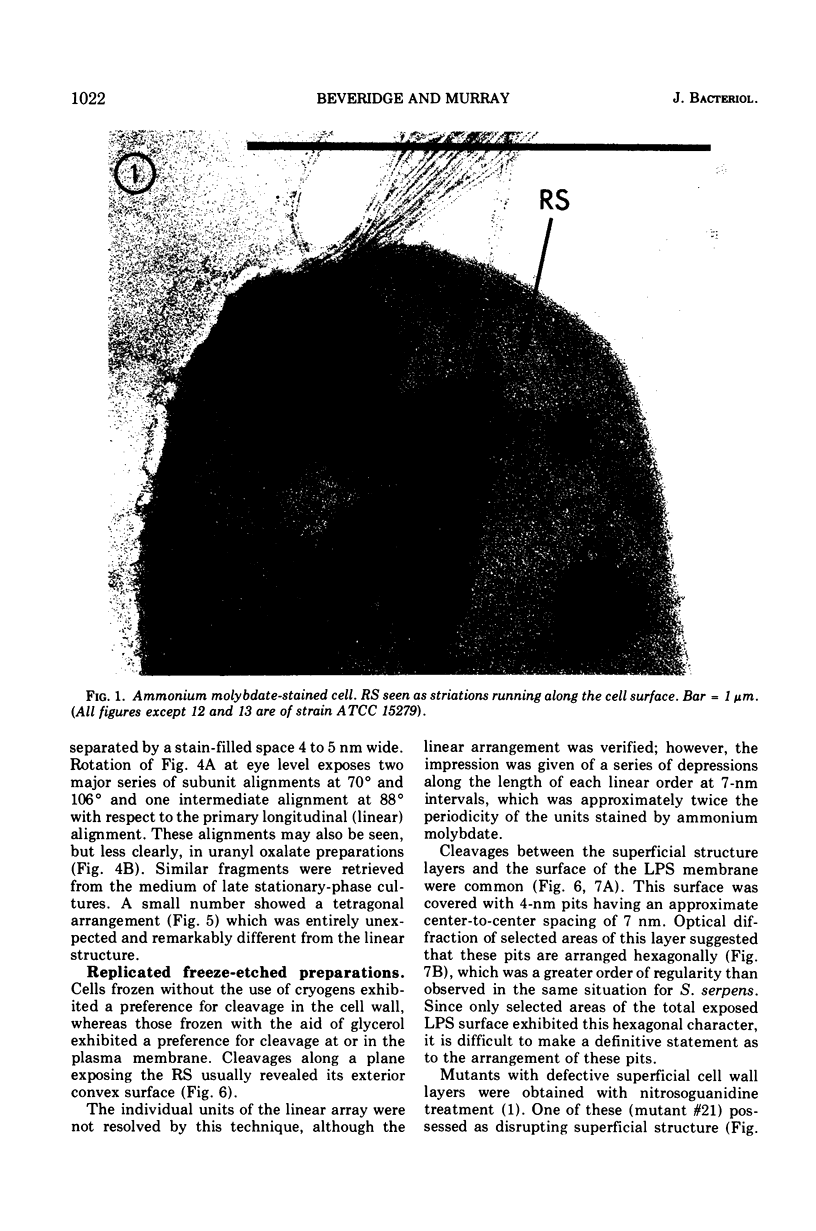
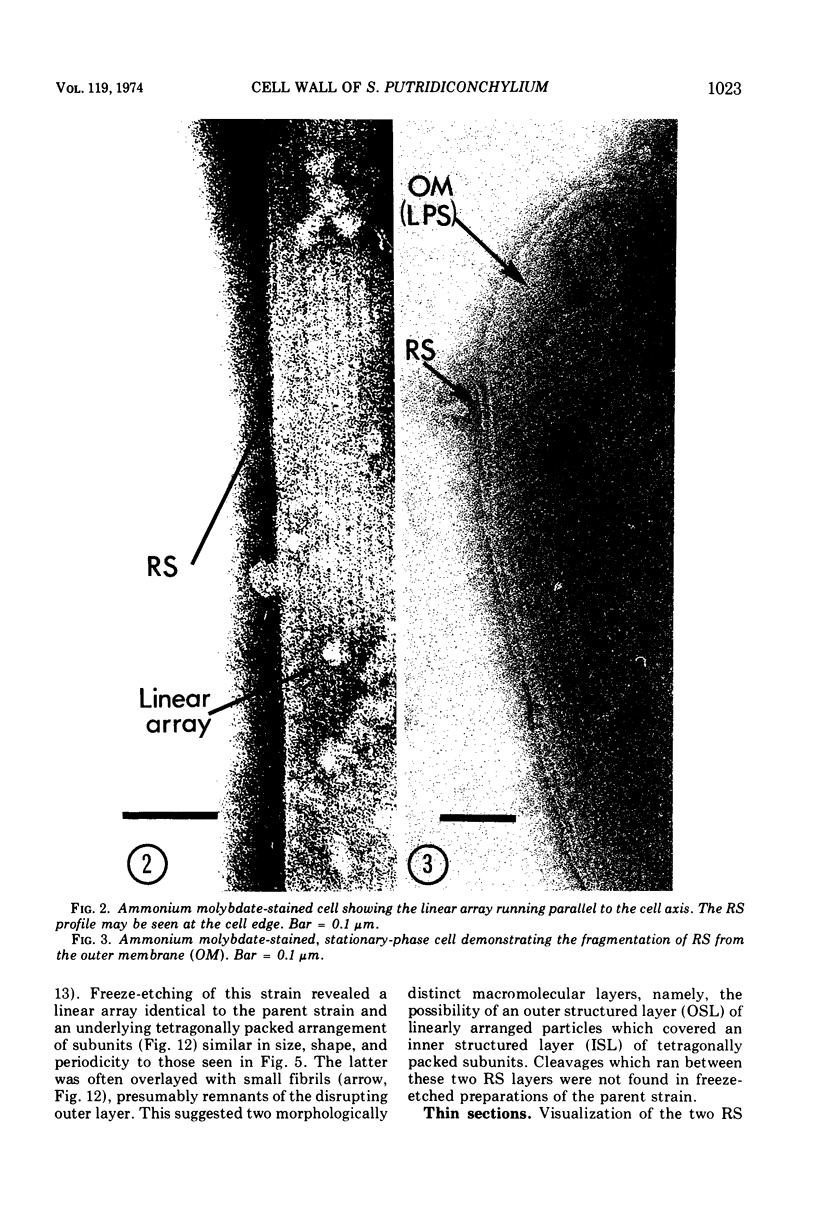
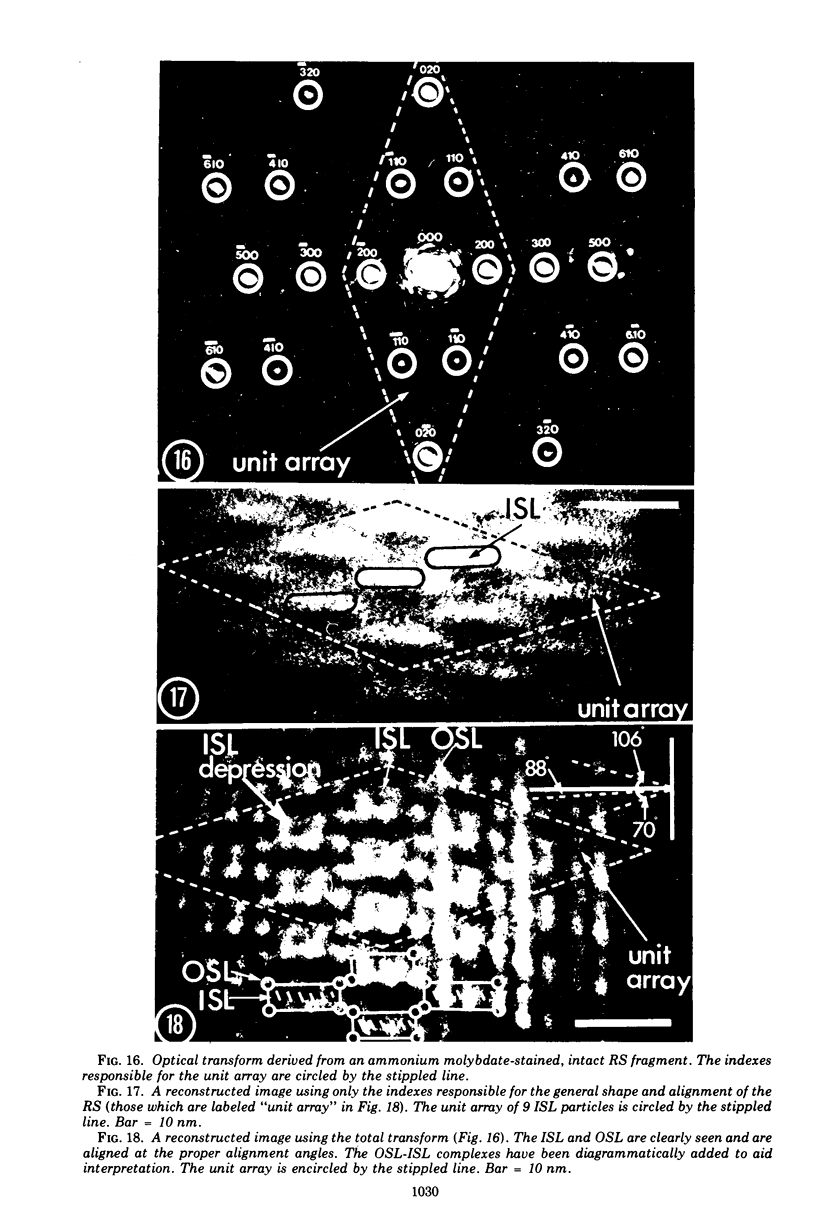
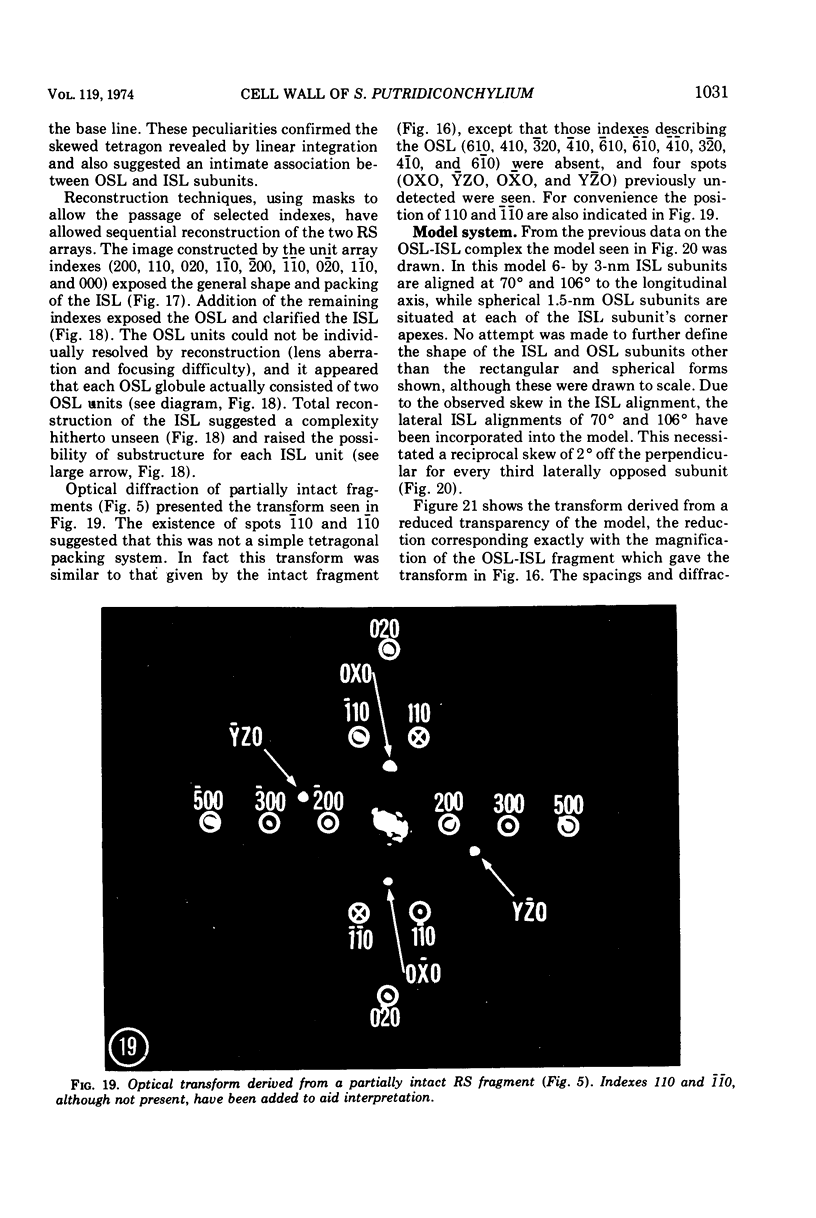
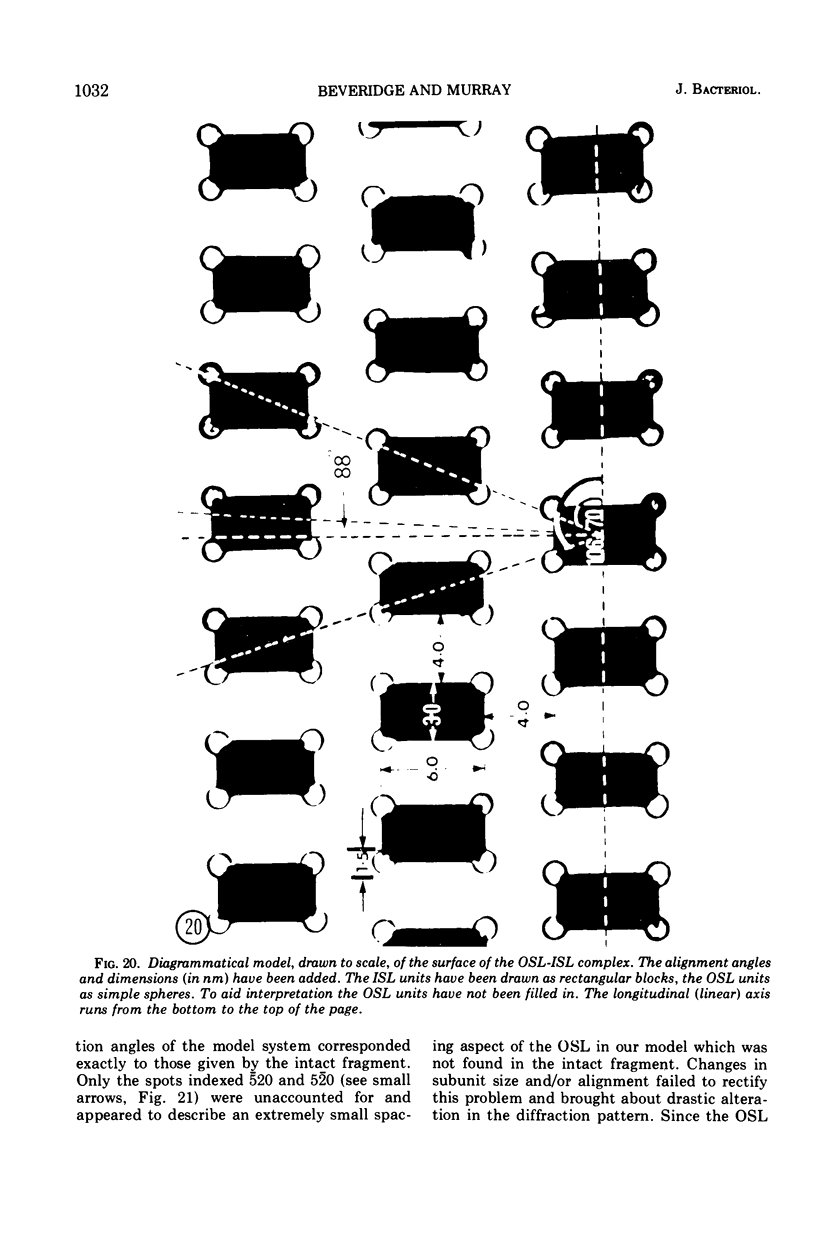
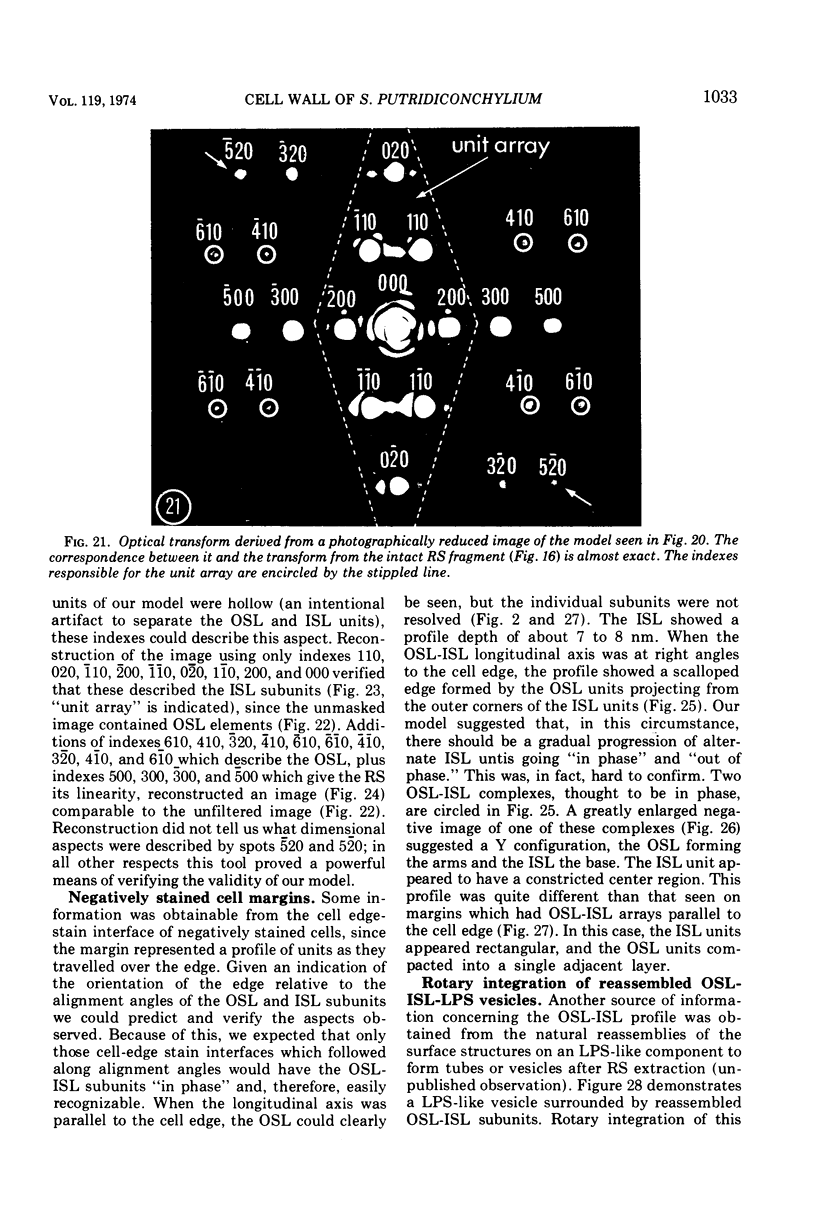
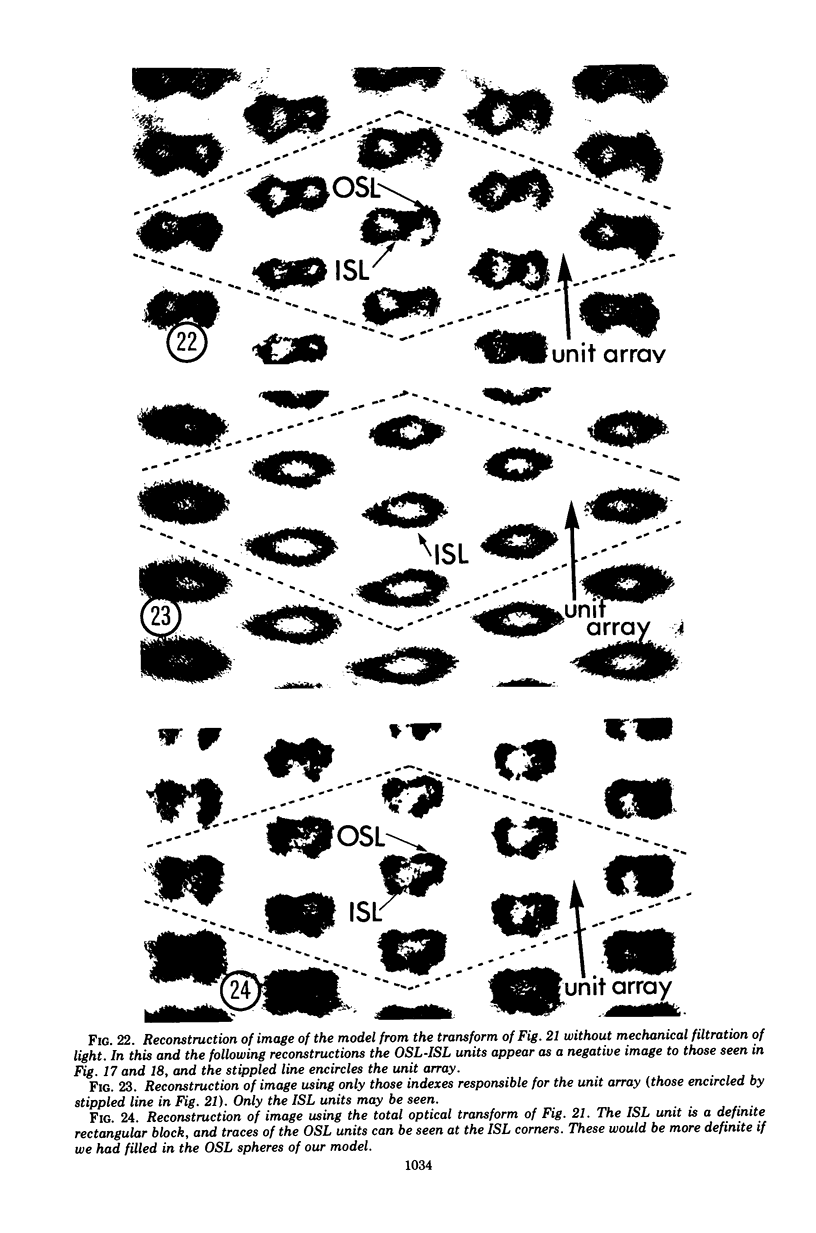
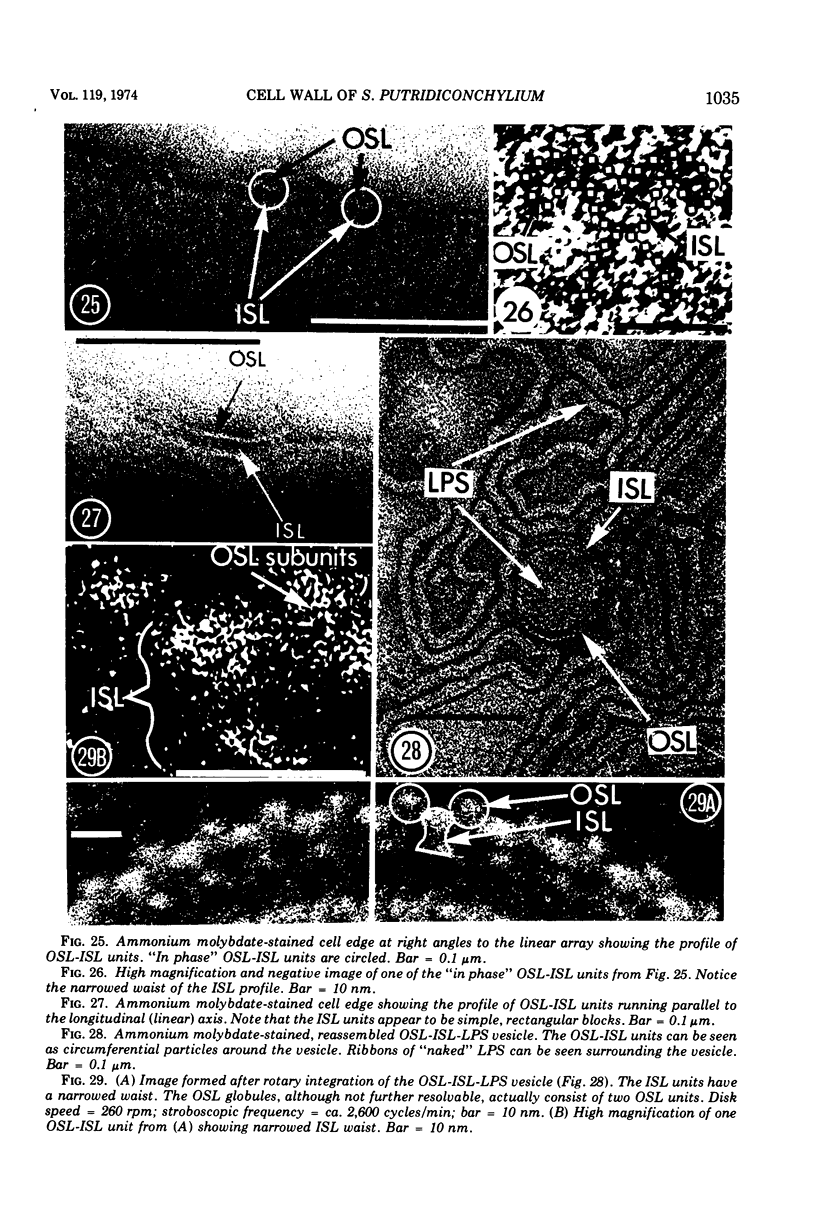
Electron microscopy of the cell envelope of Spirillum putridiconchylium, using negatively stained, thin-sectioned, and replicated freeze-etched preparations, showed two superficial wall layers forming a complex macromolecular pattern on the external surface. The outer structured layer was a linear array of particles overlying an inner tetragonal array of larger subunits. They were associated in a very regular fashion, and the complex was bonded to the outer, pitted surface of the lipopolysaccharide tripartite layer of the cell wall. The relationship of the components of the two structured layers was resolved with the aid of optical diffraction, combined with image filtering and reconstruction and linear and rotary integration techniques. The outer structural layer consisted of spherical 1.5-nm units set in double lines determined by the size and arrangement of 6- by 3-nm inner structural layer subunits, which bore one outer structural layer unit on each outer corner. The total effect of this arrangement was a double-ridged linear structure that was evident in surface replicas and negatively stained fragments of the whole wall. The packing of these units was not square but skewed by 2° off the perpendicular so that the “unit array” described by optical diffraction and linear integration appeared to be a deformed tetragon. The verity of the model was checked by using a photographically reduced image to produce an optical diffraction pattern for comparison with that of the actual layers. The correspondence was nearly perfect.
Full text
PDF


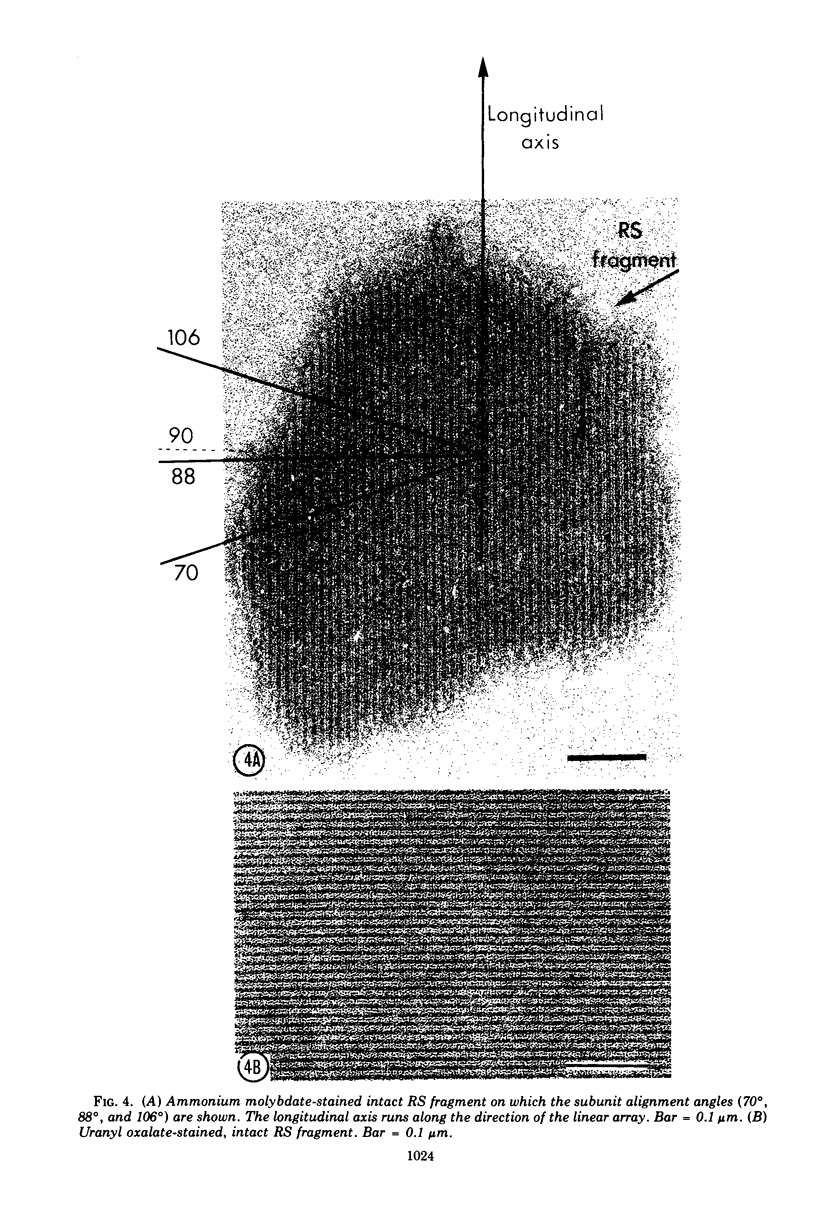
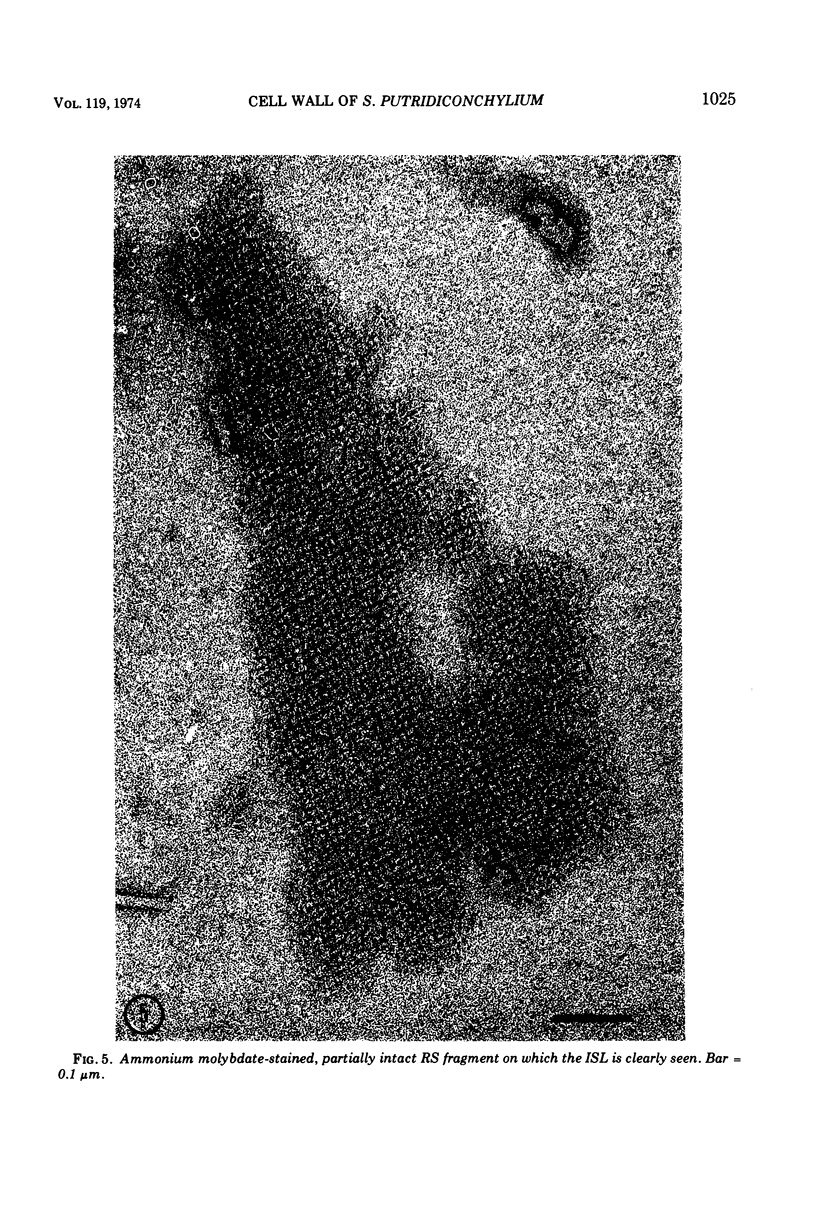
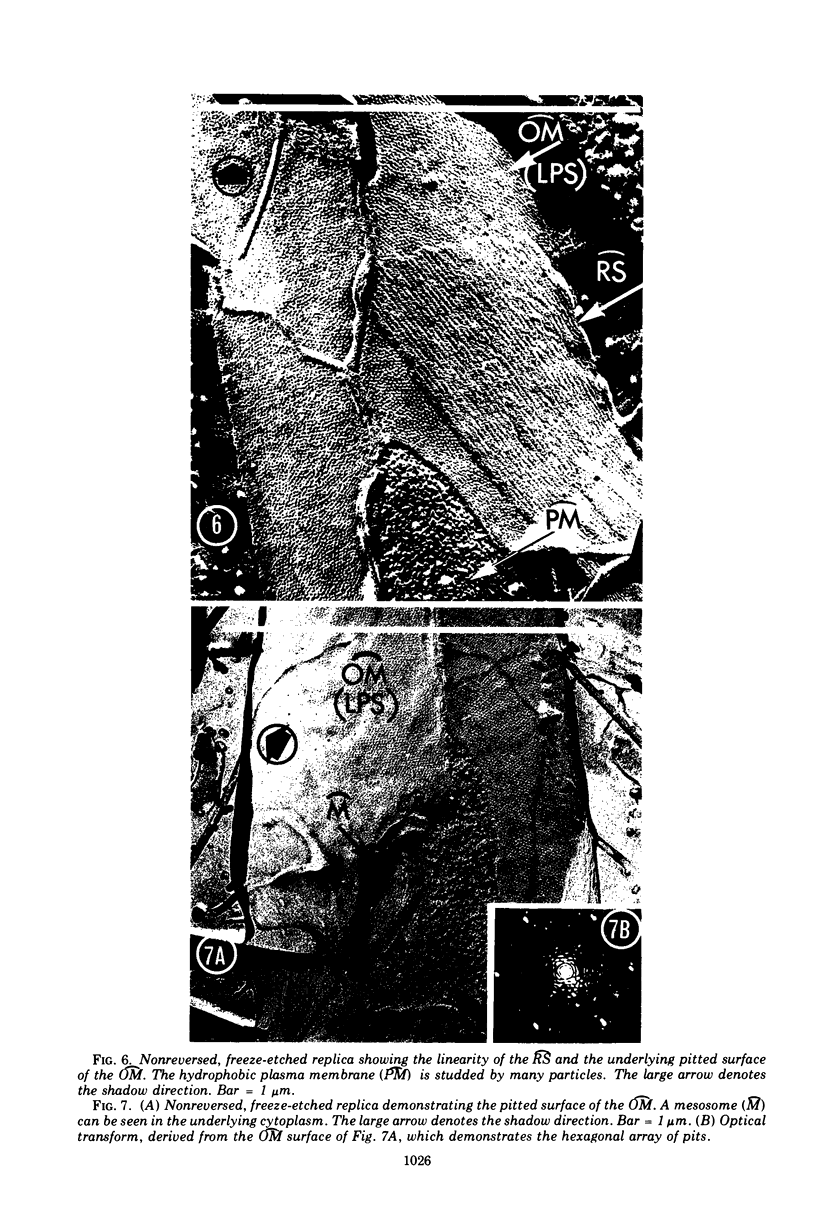

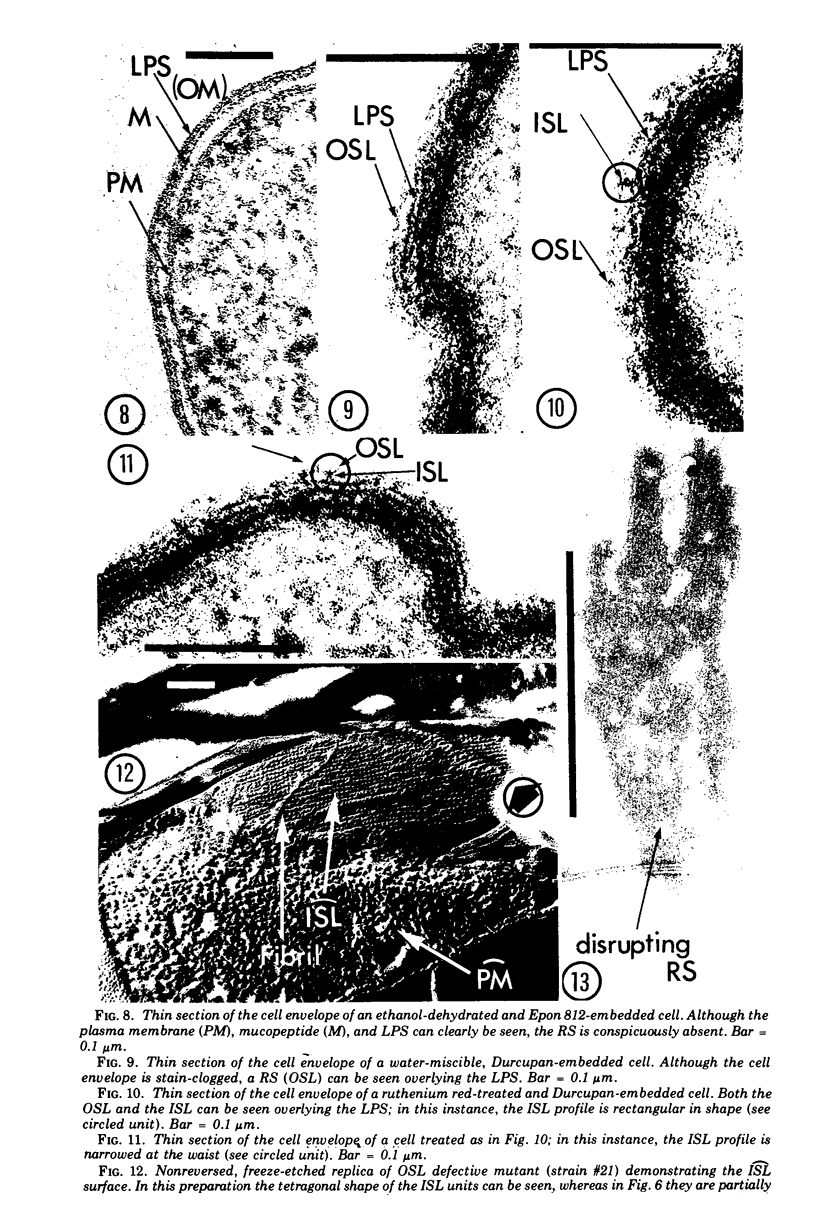




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun V., Bosch V. Sequence of the murein-lipoprotein and the attachment site of the lipid. Eur J Biochem. 1972 Jun 23;28(1):51–69. doi: 10.1111/j.1432-1033.1972.tb01883.x. [DOI] [PubMed] [Google Scholar]
- Braun V., Gnirke H., Henning U., Rehn K. Model for the structure of the shape-maintaining layer of the Escherichia coli cell envelope. J Bacteriol. 1973 Jun;114(3):1264–1270. doi: 10.1128/jb.114.3.1264-1270.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun V., Sieglin U. The covalent murein-lipoprotein structure of the Escherichia coli cell wall. The attachment site of the lipoprotein on the murein. Eur J Biochem. 1970 Apr;13(2):336–346. doi: 10.1111/j.1432-1033.1970.tb00936.x. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. 1. Isolation and partial purification of the outermost cell wall layer. Can J Microbiol. 1970 Oct;16(10):1011–1022. doi: 10.1139/m70-171. [DOI] [PubMed] [Google Scholar]
- Buckmire F. L., Murray R. G. Studies on the cell wall of Spirillum serpens. II. Chemical characterization of the outer structured layer. Can J Microbiol. 1973 Jan;19(1):59–66. doi: 10.1139/m73-009. [DOI] [PubMed] [Google Scholar]
- CHAPMAN J. A., MURRAY R. G., SALTON M. R. THE SURFACE ANATOMY OF LAMPROPEDIA HYALINA. Proc R Soc Lond B Biol Sci. 1963 Nov 19;158:498–513. doi: 10.1098/rspb.1963.0060. [DOI] [PubMed] [Google Scholar]
- De Martino C., Zamboni L. Silver methenamine stain for electron microscopy. J Ultrastruct Res. 1967 Aug;19(3):273–282. doi: 10.1016/s0022-5320(67)80221-1. [DOI] [PubMed] [Google Scholar]
- Ellar D. J., Lundgren D. G. Orded substructure in the cell wall of Bacillus cereus. J Bacteriol. 1967 Nov;94(5):1778–1780. doi: 10.1128/jb.94.5.1778-1780.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedman M. H. A reevaluation of the Markham rotation technique using model systems. J Ultrastruct Res. 1970 Aug;32(3):226–236. doi: 10.1016/s0022-5320(70)80003-x. [DOI] [PubMed] [Google Scholar]
- Glauert A. M., Thornley M. J. The topography of the bacterial cell wall. Annu Rev Microbiol. 1969;23:159–198. doi: 10.1146/annurev.mi.23.100169.001111. [DOI] [PubMed] [Google Scholar]
- Harris J. R., Agutter P. A negative staining study of human erythrocyte ghosts and rat liver nuclear membranes. J Ultrastruct Res. 1970 Nov;33(3):219–232. doi: 10.1016/s0022-5320(70)90017-1. [DOI] [PubMed] [Google Scholar]
- Holt S. C., Leadbetter E. R. Comparative ultrastructure of selected aerobic spore-forming bacteria: a freeze-etching study. Bacteriol Rev. 1969 Jun;33(2):346–378. doi: 10.1128/br.33.2.346-378.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard L., Tipper D. J. A polypeptide bacteriophage receptor: modified cell wall protein subunits in bacteriophage-resistant mutants of Bacillus sphaericus strain P-1. J Bacteriol. 1973 Mar;113(3):1491–1504. doi: 10.1128/jb.113.3.1491-1504.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KLUG A., BERGER J. E. AN OPTICAL METHOD FOR THE ANALYSIS OF PERIODICITIES IN ELECTRON MICROGRAPHS, AND SOME OBSERVATIONS ON THE MECHANISM OF NEGATIVE STAINING. J Mol Biol. 1964 Dec;10:565–569. doi: 10.1016/s0022-2836(64)80081-4. [DOI] [PubMed] [Google Scholar]
- Klug A., De Rosier D. J. Optical filtering of electron micrographs: reconstruction of one-sided images. Nature. 1966 Oct 1;212(5057):29–32. doi: 10.1038/212029a0. [DOI] [PubMed] [Google Scholar]
- LUFT J. H. Improvements in epoxy resin embedding methods. J Biophys Biochem Cytol. 1961 Feb;9:409–414. doi: 10.1083/jcb.9.2.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leduc E. H., Holt S. J. Hydroxypropyl methacrylate, a new water-miscible embedding medium for electron microscopy. J Cell Biol. 1965 Jul;26(1):137–155. doi: 10.1083/jcb.26.1.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft J. H. Ruthenium red and violet. I. Chemistry, purification, methods of use for electron microscopy and mechanism of action. Anat Rec. 1971 Nov;171(3):347–368. doi: 10.1002/ar.1091710302. [DOI] [PubMed] [Google Scholar]
- MARKHAM R., HITCHBORN J. H., HILLS G. J., FREY S. THE ANATOMY OF THE TOBACCO MOSAIC VIRUS. Virology. 1964 Mar;22:342–359. doi: 10.1016/0042-6822(64)90025-x. [DOI] [PubMed] [Google Scholar]
- McElroy L. J., Krieg N. R. A serological method for the identification of Spirilla. Can J Microbiol. 1972 Jan;18(1):57–64. doi: 10.1139/m72-009. [DOI] [PubMed] [Google Scholar]
- Mellema J. E., van Bruggen E. F. An assessment of negative staining in the electron microscopy of low molecular weight proteins. J Mol Biol. 1968 Jan 14;31(1):75–82. doi: 10.1016/0022-2836(68)90055-7. [DOI] [PubMed] [Google Scholar]
- Nermut M. V., Murray R. G. Ultrastructure of the cell wall of Bacillus polymyxa. J Bacteriol. 1967 Jun;93(6):1949–1965. doi: 10.1128/jb.93.6.1949-1965.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- REYNOLDS E. S. The use of lead citrate at high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963 Apr;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A., KELLENBERGER E. L'inclusion au polyester pour l'ultramicrotomie. J Ultrastruct Res. 1958 Dec;2(2):200–214. doi: 10.1016/s0022-5320(58)90018-2. [DOI] [PubMed] [Google Scholar]
- Reyn A., Birch-Andersen A., Murray R. G. The fine structure of Cardiobacterium hominis. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(1):51–60. doi: 10.1111/j.1699-0463.1971.tb00032.x. [DOI] [PubMed] [Google Scholar]
- Scott J. E., Quintarelli G., Dellovo M. C. The chemical and histochemical properties of Alcian Blue. I. The mechanism of Alcian Blue staining. Histochemie. 1964 Jul 17;4(2):73–85. doi: 10.1007/BF00306149. [DOI] [PubMed] [Google Scholar]
- Sleytr U. B., Thornley M. J. Freeze-etching of the cell envelope of an Acinetobacter species which carries a regular array of surface subunits. J Bacteriol. 1973 Dec;116(3):1383–1397. doi: 10.1128/jb.116.3.1383-1397.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorne K. J., Thornley M. J., Glauert A. M. Chemical analysis of the outer membrane and other layers of the cell envelope of Acinetobacter sp. J Bacteriol. 1973 Oct;116(1):410–417. doi: 10.1128/jb.116.1.410-417.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M. Fine structure and radiation resistance in Acinetobacter: studies on a resistant strain. J Cell Sci. 1968 Jun;3(2):273–294. doi: 10.1242/jcs.3.2.273. [DOI] [PubMed] [Google Scholar]
- Thornley M. J., Glauert A. M., Sleytr U. B. Isolation of outer membranes with an ordered array of surface subunits from Acinetobacter. J Bacteriol. 1973 Jun;114(3):1294–1308. doi: 10.1128/jb.114.3.1294-1308.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson S. W., Remsen C. C. Cell envelope of Nitrosocystis oceanus. J Ultrastruct Res. 1970 Oct;33(1):148–160. doi: 10.1016/s0022-5320(70)90122-x. [DOI] [PubMed] [Google Scholar]