Abstract
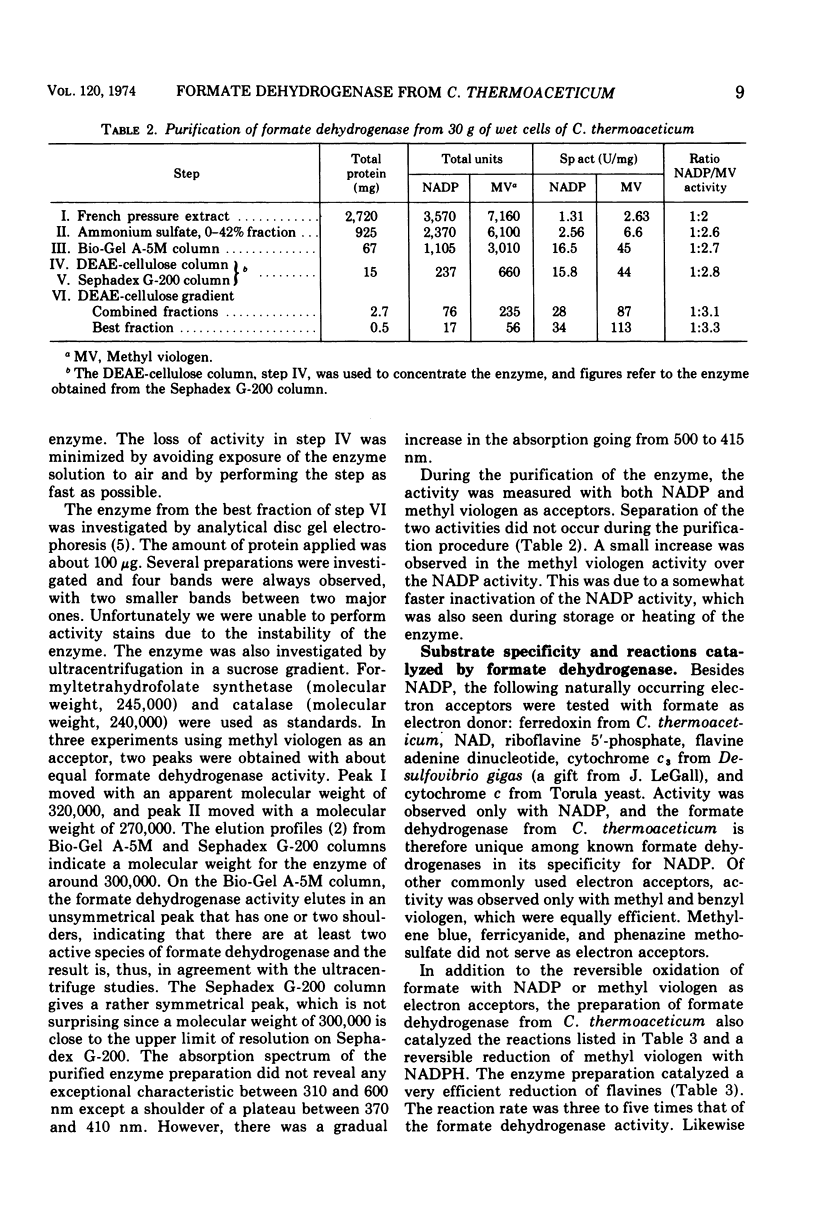
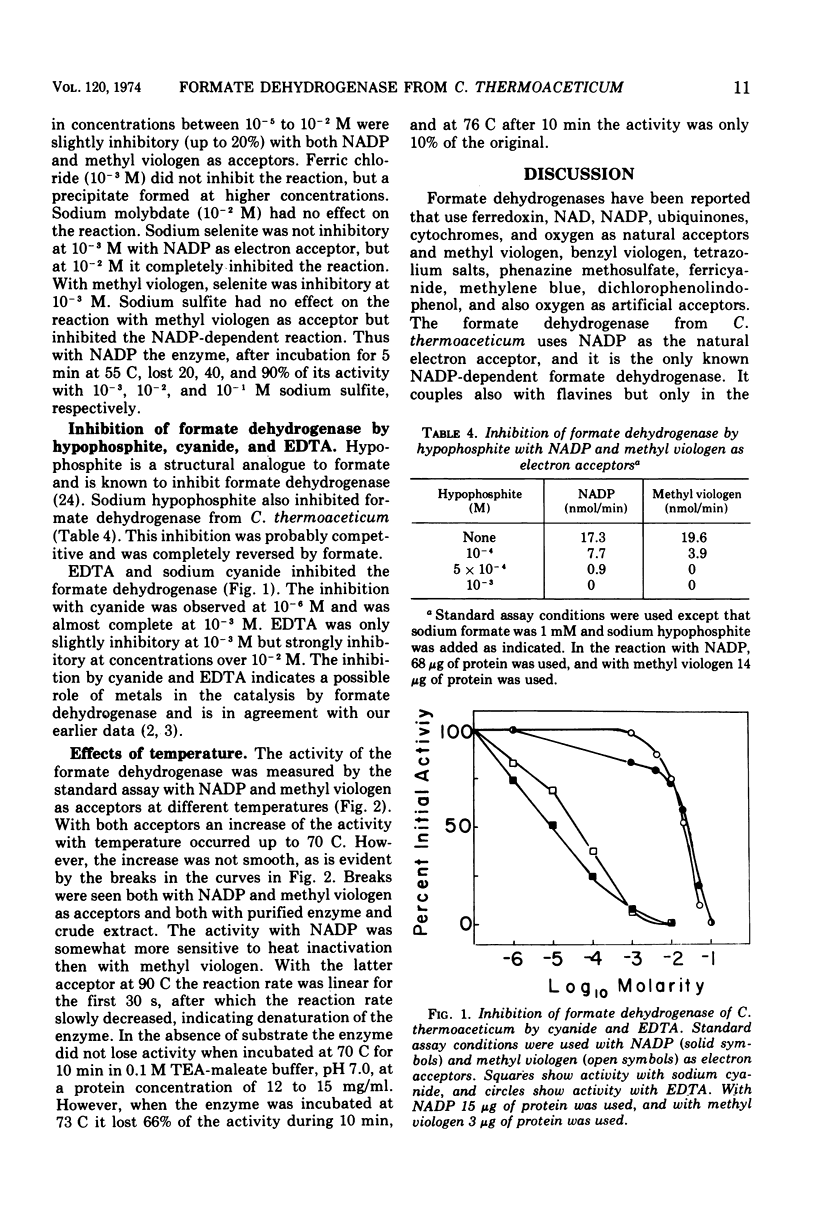
The nicotinamide adenine dinucleotide phosphate (NADP)-dependent formate dehydrogenase in Clostridium thermoaceticum used, in addition to its natural electron acceptor, methyl and benzyl viologen. The enzyme was purified to a specific activity of 34 (micromoles per minute per milligram of protein) with NADP as electron acceptor. Disc gel electrophoresis of the purified enzyme yielded two major and two minor protein bands, and during centrifugation in sucrose gradients two components of apparent molecular weights of 270,000 and 320,000 were obtained, both having formate dehydrogenase activity. The enzyme preparation catalyzed the reduction of riboflavine 5′-phosphate flavine adenine dinucleotide and methyl viologen by using reduced NADP as a source of electrons. It also had reduced NADP oxidase activity. The enzyme was strongly inhibited by cyanide and ethylenediaminetetraacetic acid. It was also inhibited by hypophosphite, an inhibition that was reversed by formate. Sulfite inhibited the activity with NADP but not with methyl viologen as acceptor. The apparent Km at 55 C and pH 7.5 for formate was 2.27 × 10−4 M with NADP and 0.83 × 10−4 with methyl viologen as acceptor. The apparent Km for NADP was 1.09 × 10−4 M and for methyl viologen was 2.35 × 10−3 M. NADP showed substrate inhibition at 5 × 10−3 M and higher concentrations. With NADP as electron acceptor, the enzyme had a broad pH optimum between 7 and 9.5. The apparent temperature optimum was 85 C. In the absence of substrates, the enzyme was stable at 70 C but was rapidly inactivated at temperatures above 73 C. The enzyme was very sensitive to oxygen but was stabilized by thiol-iron complexes and formate.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andreesen J. R., El Ghazzawi E., Gottschalk G. The effect of ferrous ions, tungstate and selenite on the level of formate dehydrogenase in Clostridium formicoaceticum and formate synthesis from CO2 during pyruvate fermentation. Arch Mikrobiol. 1974 Mar 4;96(2):103–118. doi: 10.1007/BF00590167. [DOI] [PubMed] [Google Scholar]
- Andreesen J. R., Ljungdahl L. G. Formate dehydrogenase of Clostridium thermoaceticum: incorporation of selenium-75, and the effects of selenite, molybdate, and tungstate on the enzyme. J Bacteriol. 1973 Nov;116(2):867–873. doi: 10.1128/jb.116.2.867-873.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreesen J. R., Schaupp A., Neurauter C., Brown A., Ljungdahl L. G. Fermentation of glucose, fructose, and xylose by Clostridium thermoaceticum: effect of metals on growth yield, enzymes, and the synthesis of acetate from CO 2 . J Bacteriol. 1973 May;114(2):743–751. doi: 10.1128/jb.114.2.743-751.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer J. M., Ashworth R. B. Disc electrophoresis. J Chem Educ. 1969 Jan;46(1):41–45. doi: 10.1021/ed046p41. [DOI] [PubMed] [Google Scholar]
- Böger P. Zusammenhang zwischen Transhydrogenase- und Diaphorase-Akvivität der Ferredoxin-NADP-Reduktase und der photosynthetischen NADP-Reduktion. Z Naturforsch B. 1971 Aug;26(8):807–815. doi: 10.1515/znb-1971-0815. [DOI] [PubMed] [Google Scholar]
- Corwin A. H., Arellano R. R., Chivvis A. B. Anomalies of viologens in bases and water. Biochim Biophys Acta. 1968 Nov 26;162(4):533–538. doi: 10.1016/0005-2728(68)90060-1. [DOI] [PubMed] [Google Scholar]
- Coughlan M. P., Rajagopalan K. V., Handler P. The role of molybdenum in xanthine oxidase and related enzymes. Reactivity with cyanide, arsenite, and methanol. J Biol Chem. 1969 May 25;244(10):2658–2663. [PubMed] [Google Scholar]
- De Lumen B. O., Tappel A. L. Fluorescein-hemoglobin as a substrate for cathepsin D and other proteases. Anal Biochem. 1970 Jul;36(1):22–29. doi: 10.1016/0003-2697(70)90328-3. [DOI] [PubMed] [Google Scholar]
- Enoch H. G., Lester R. L. Effects of molybdate, tungstate, and selenium compounds on formate dehydrogenase and other enzyme systems in Escherichia coli. J Bacteriol. 1972 Jun;110(3):1032–1040. doi: 10.1128/jb.110.3.1032-1040.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enzymatic -oxidation. VI. Isolation of homogeneous reduced diphosphopyridine nucleotide-rubredoxin reductase. J Biol Chem. 1972 Apr 10;247(7):2109–2116. [PubMed] [Google Scholar]
- Fontaine F. E., Peterson W. H., McCoy E., Johnson M. J., Ritter G. J. A New Type of Glucose Fermentation by Clostridium thermoaceticum. J Bacteriol. 1942 Jun;43(6):701–715. doi: 10.1128/jb.43.6.701-715.1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forget P. The bacterial nitrate reductases. Solubilization, purification and properties of the enzyme A of Escherichia coli K 12. Eur J Biochem. 1974 Mar 1;42(2):325–332. doi: 10.1111/j.1432-1033.1974.tb03343.x. [DOI] [PubMed] [Google Scholar]
- Ghambeer R. K., Wood H. G., Schulman M., Ljungdahl L. Total synthesis of acetate from CO2. 3. Inhibition by alkylhalides of the synthesis from CO2, methyltetrahydrofolate, and methyl-B12 by Clostridium thermoaceticum. Arch Biochem Biophys. 1971 Apr;143(2):471–484. doi: 10.1016/0003-9861(71)90232-3. [DOI] [PubMed] [Google Scholar]
- Holcenberg J. S., Stadtman E. R. Nicotinic acid metabolism. 3. Purification and properties of a nicotinic acid hydroxylase. J Biol Chem. 1969 Mar 10;244(5):1194–1203. [PubMed] [Google Scholar]
- Höpner T., Trautwein A. Some properties of formate dehydrogenase. Z Naturforsch B. 1972 Sep;27(9):1075–1076. doi: 10.1515/znb-1972-0923. [DOI] [PubMed] [Google Scholar]
- Jungermann K., Kirchniawy H., Thauer R. K. Ferredoxin dependent CO-2 reduction to formate in Clostridium pasteurianum. Biochem Biophys Res Commun. 1970 Nov 9;41(3):682–689. doi: 10.1016/0006-291x(70)90067-7. [DOI] [PubMed] [Google Scholar]
- Kearny J. J., Sagers R. D. Formate dehydrogenase from Clostridium acidiurici. J Bacteriol. 1972 Jan;109(1):152–161. doi: 10.1128/jb.109.1.152-161.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LENTZ K., WOOD H. G. Synthesis of acetate from formate and carbon dioxide by Clostridium thermoaceticum. J Biol Chem. 1955 Aug;215(2):645–654. [PubMed] [Google Scholar]
- Li L. F., Ljungdahl L., Wood H. G. Properties of Nicotinamide Adenine Dinucleotide Phosphate-Dependent Formate Dehydrogenase from Clostridium thermoaceticum. J Bacteriol. 1966 Aug;92(2):405–412. doi: 10.1128/jb.92.2.405-412.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ljungdahl L. G. Total synthesis of acetate from CO2 by heterotrophic bacteria. Annu Rev Microbiol. 1969;23:515–538. doi: 10.1146/annurev.mi.23.100169.002503. [DOI] [PubMed] [Google Scholar]
- Ljungdahl L., Irion E., Wood H. G. Total synthesis of acetate from CO2. I. Co-methylcobyric acid and CO-(methyl)-5-methoxybenzimidazolylcobamide as intermediates with Clostridium thermoaceticum. Biochemistry. 1965 Dec;4(12):2771–2780. doi: 10.1021/bi00888a030. [DOI] [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- O'Brien W. E., Ljungdahl L. G. Fermentation of fructose and synthesis of acetate from carbon dioxide by Clostridium formicoaceticum. J Bacteriol. 1972 Feb;109(2):626–632. doi: 10.1128/jb.109.2.626-632.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PEEL J. L. THE CATALYSIS OF THE AUTO-OXIDATION OF 2-MERCAPTOETHANOL AND OTHER THIOLS BY VITAMIN B12 DERIVATIVES. POLAROGRAPHIC AND OTHER INVESTIGATIONS. Biochem J. 1963 Aug;88:296–308. doi: 10.1042/bj0880296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINE M. J. Methane fermentation of formate by Methanobacillus omelianskii. J Bacteriol. 1958 Mar;75(3):356–359. doi: 10.1128/jb.75.3.356-359.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PINSENT J. The need for selenite and molybdate in the formation of formic dehydrogenase by members of the coli-aerogenes group of bacteria. Biochem J. 1954 May;57(1):10–16. doi: 10.1042/bj0570010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulman M., Parker D., Ljungdahl L. G., Wood H. G. Total synthesis of acetate from CO 2 . V. Determination by mass analysis of the different types of acetate formed from 13 CO 2 by heterotrophic bacteria. J Bacteriol. 1972 Feb;109(2):633–644. doi: 10.1128/jb.109.2.633-644.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shum A. C., Murphy J. C. Effects of selenium compounds on formate metabolism and coincidence of selenium-75 incorporation and formic dehydrogenase activity in cell-free preparations of Escherichia coli. J Bacteriol. 1972 Apr;110(1):447–449. doi: 10.1128/jb.110.1.447-449.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadtman T. C. Selenium biochemistry. Science. 1974 Mar 8;183(4128):915–922. doi: 10.1126/science.183.4128.915. [DOI] [PubMed] [Google Scholar]
- Thauer R. K. CO(2)-reduction to formate by NADPH. The initial step in the total synthesis of acetate from CO(2) in Clostridium thermoaceticum. FEBS Lett. 1972 Oct 15;27(1):111–115. doi: 10.1016/0014-5793(72)80421-6. [DOI] [PubMed] [Google Scholar]
- Thauer R. K., Fuchs G., Schnitker U., Jungermann K. CO2 reductase from Clostridium pasteurianum: molybdenum dependence of synthesis and inactivation by cyanide. FEBS Lett. 1973 Dec 15;38(1):45–48. doi: 10.1016/0014-5793(73)80509-5. [DOI] [PubMed] [Google Scholar]
- Turner D. C., Stadtman T. C. Purification of protein components of the clostridial glycine reductase system and characterization of protein A as a selenoprotein. Arch Biochem Biophys. 1973 Jan;154(1):366–381. doi: 10.1016/0003-9861(73)90069-6. [DOI] [PubMed] [Google Scholar]
- WOOD H. G. A study of carbon dioxide fixation by mass determination of the types of C13-acetate. J Biol Chem. 1952 Feb;194(2):905–931. [PubMed] [Google Scholar]
- Yoch D. C. Purification and characterization of ferredoxin-nicotinamide adenine dinucleotide phosphate reductase from a nitrogen-fixing bacterium. J Bacteriol. 1973 Oct;116(1):384–391. doi: 10.1128/jb.116.1.384-391.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti G., Forti G. Studies on the triphosphopyridine nucleotide-cytochrome f reductase of chloroplasts. J Biol Chem. 1966 Jan 25;241(2):279–285. [PubMed] [Google Scholar]


