Abstract
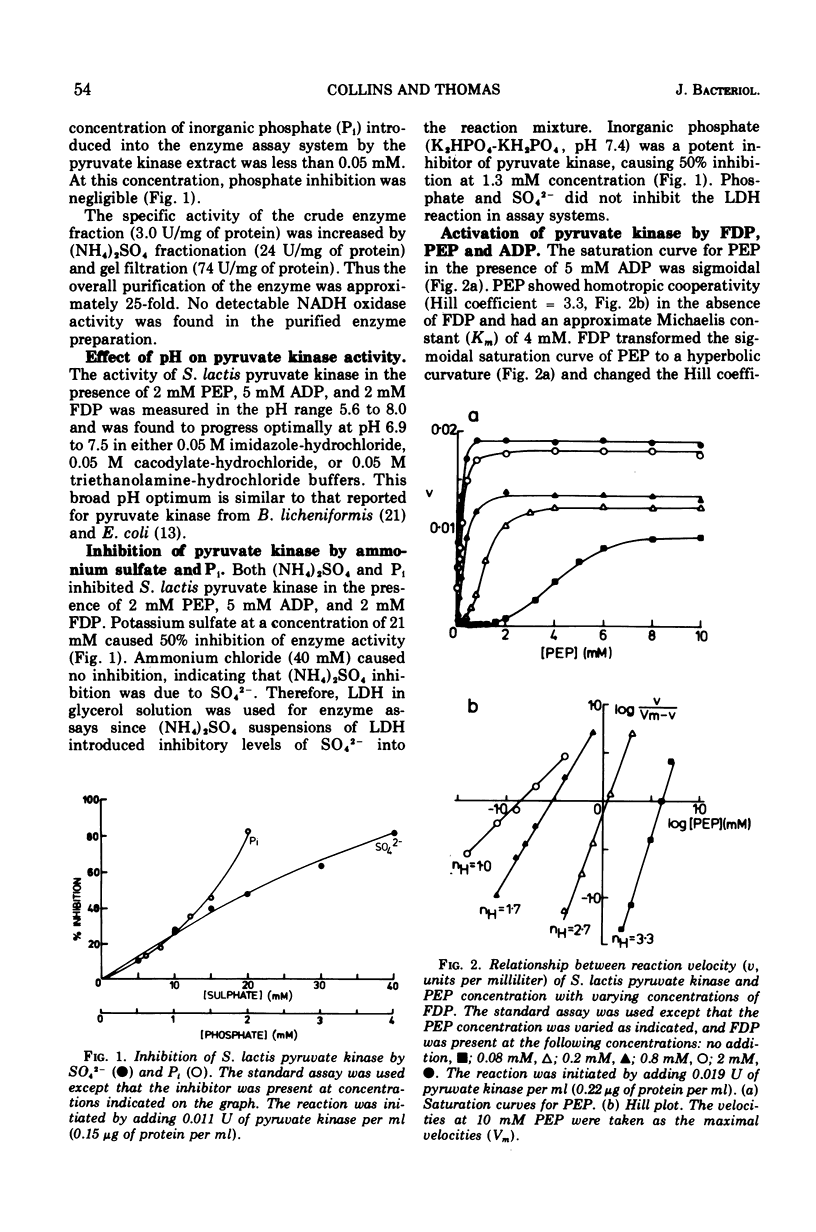
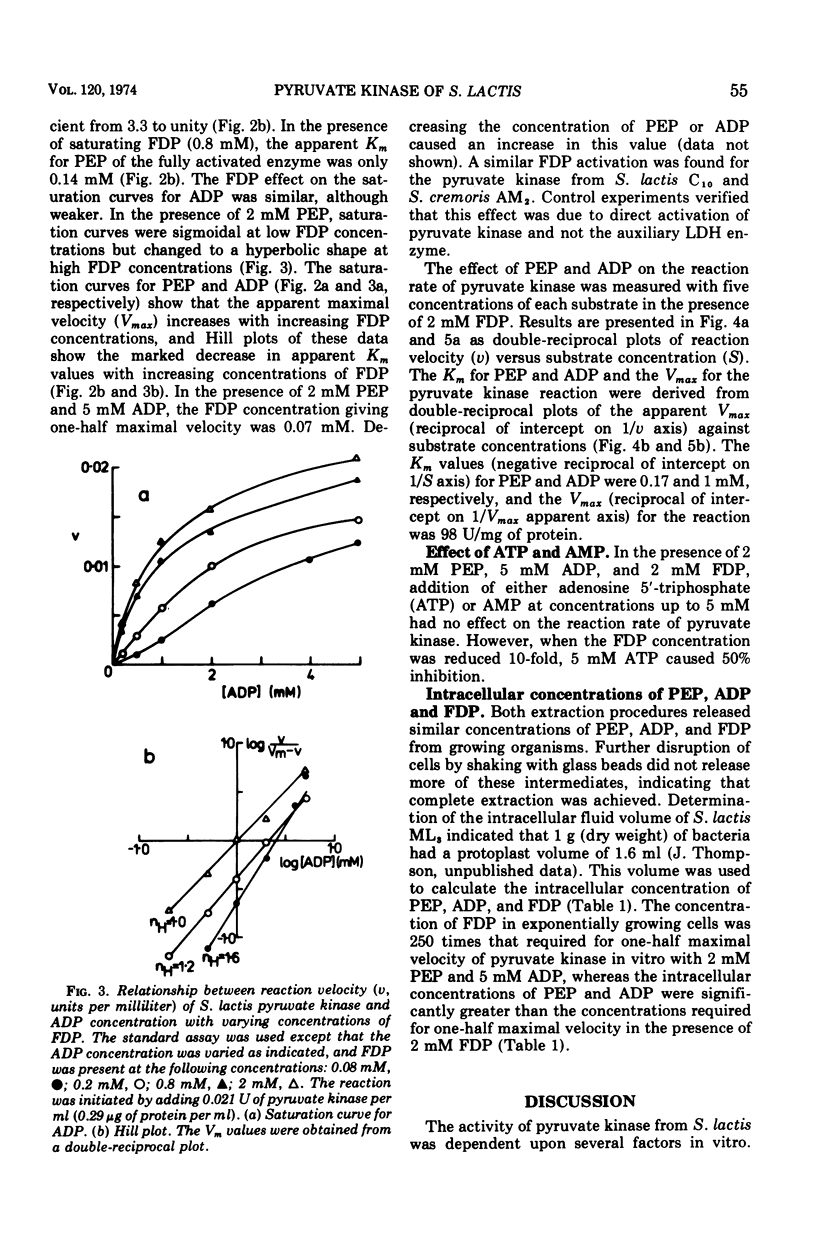
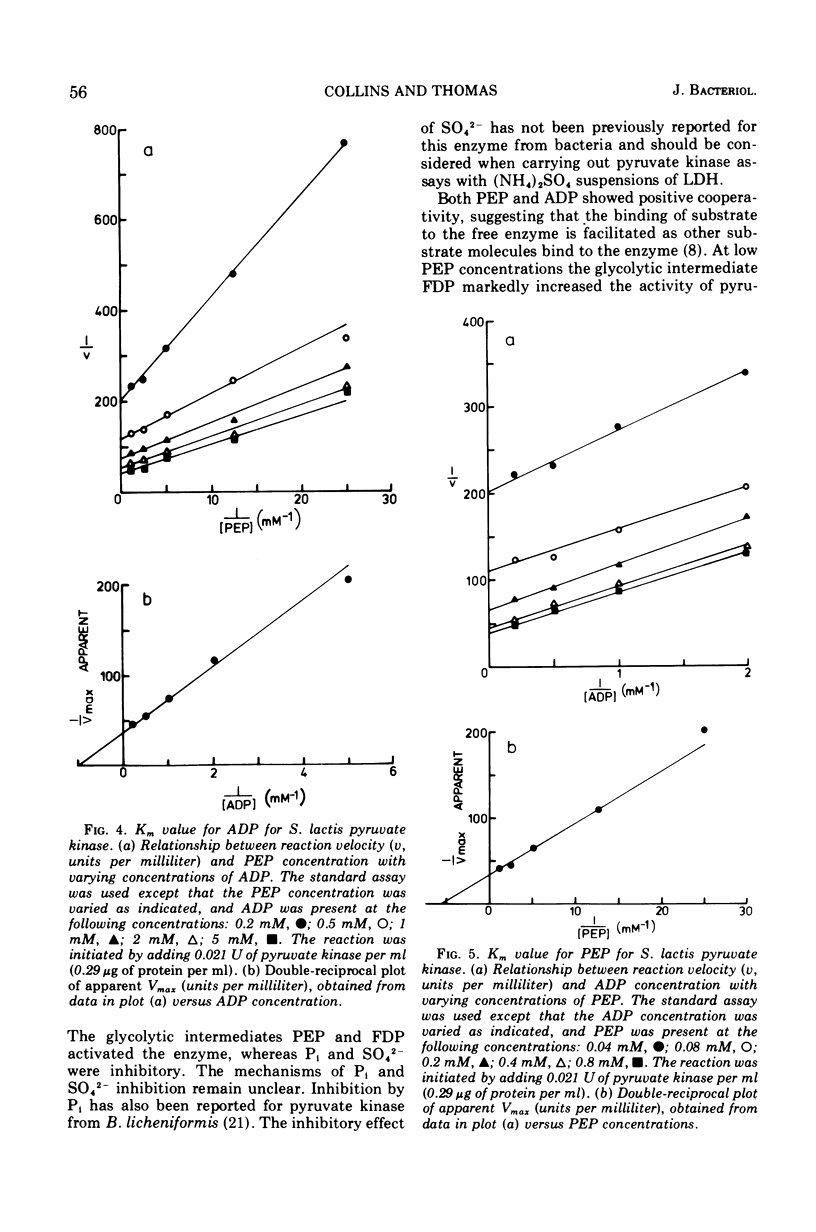
The kinetic properties of pyruvate kinase (ATP:pyruvate-phosphotransferase, EC 2.7.1.40) from Streptococcus lactis have been investigated. Positive homotropic kinetics were observed with phosphoenolpyruvate and adenosine 5′-diphosphate, resulting in a sigmoid relationship between reaction velocity and substrate concentrations. This relationship was abolished with an excess of the heterotropic effector fructose-1,6-diphosphate, giving a typical Michaelis-Menten relationship. Increasing the concentration of fructose-1,6-diphosphate increased the apparent Vmax values and decreased the Km values for both substrates. Catalysis by pyruvate kinase proceeded optimally at pH 6.9 to 7.5 and was markedly inhibited by inorganic phosphate and sulfate ions. Under certain conditions adenosine 5′-triphosphate also caused inhibition. The Km values for phosphoenolpyruvate and adenosine 5′-diphosphate in the presence of 2 mM fructose-1,6-diphosphate were 0.17 mM and 1 mM, respectively. The concentration of fructose-1,6-diphosphate giving one-half maximal velocity with 2 mM phosphoenolpyruvate and 5 mM adenosine 5′-diphosphate was 0.07 mM. The intracellular concentrations of these metabolites (0.8 mM phosphoenolpyruvate, 2.4 mM adenosine 5′-diphosphate, and 18 mM fructose-1,6-diphosphate) suggest that the pyruvate kinase in S. lactis approaches maximal activity in exponentially growing cells. The role of pyruvate kinase in the regulation of the glycolytic pathway in lactic streptococci is discussed.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BLACK S. H., GERHARDT P. Permeability of bacterial spores. IV. Water content, uptake, and distribution. J Bacteriol. 1962 May;83:960–967. doi: 10.1128/jb.83.5.960-967.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diesterhaft M., Freese E. Pyruvate kinase of bacillus subtilis. Biochim Biophys Acta. 1972 May 12;268(2):373–380. doi: 10.1016/0005-2744(72)90332-4. [DOI] [PubMed] [Google Scholar]
- Gancedo J. M., Gancedo C. Concentrations of intermediary metabolites in yeast. Biochimie. 1973;55(2):205–211. doi: 10.1016/s0300-9084(73)80393-1. [DOI] [PubMed] [Google Scholar]
- Haeckel R., Hess B., Lauterborn W., Wüster K. H. Purification and allosteric properties of yeast pyruvate kinase. Hoppe Seylers Z Physiol Chem. 1968 May;349(5):699–714. doi: 10.1515/bchm2.1968.349.1.699. [DOI] [PubMed] [Google Scholar]
- Jonas H. A., Anders R. F., Jago G. R. Factors affecting the activity of the lactate dehydrognease of Streptococcus cremoris. J Bacteriol. 1972 Aug;111(2):397–403. doi: 10.1128/jb.111.2.397-403.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg H. L., Malcovati M. Control in situ of the pyruvate kinase activity of Escherichia coli. FEBS Lett. 1973 Jun 1;32(2):257–259. doi: 10.1016/0014-5793(73)80846-4. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lowry O. H., Carter J., Ward J. B., Glaser L. The effect of carbon and nitrogen sources on the level of metabolic intermediates in Escherichia coli. J Biol Chem. 1971 Nov;246(21):6511–6521. [PubMed] [Google Scholar]
- Maeba P., Sanwal B. D. The regulation of pyruvate kinase of Escherichia coli by fructose diphosphate and adenylic acid. J Biol Chem. 1968 Jan 25;243(2):448–450. [PubMed] [Google Scholar]
- Malcovati M., Kornberg H. L. Two types of pyruvate kinase in Escherichia coli K12. Biochim Biophys Acta. 1969 Apr 22;178(2):420–423. doi: 10.1016/0005-2744(69)90417-3. [DOI] [PubMed] [Google Scholar]
- McKay L. L., Walter L. A., Sandine W. E., Elliker P. R. Involvement of phosphoenolpyruvate in lactose utilization by group N streptococci. J Bacteriol. 1969 Aug;99(2):603–610. doi: 10.1128/jb.99.2.603-610.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay L., Miller A., 3rd, Sandine W. E., Elliker P. R. Mechanisms of lactose utilization by lactic acid streptococci: enzymatic and genetic analyses. J Bacteriol. 1970 Jun;102(3):804–809. doi: 10.1128/jb.102.3.804-809.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opheim D., Bernlohr R. W. Purification and regulation of glucose-6-phosphate dehydrogenase from Bacillus licheniformis. J Bacteriol. 1973 Dec;116(3):1150–1159. doi: 10.1128/jb.116.3.1150-1159.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozaki H., Shiio I. Regulation of the TCA and glyoxylate cycles in Brevibacterium flavum. II. Regulation of phosphoenolpyruvate carboxylase and pyruvate kinase. J Biochem. 1969 Sep;66(3):297–311. doi: 10.1093/oxfordjournals.jbchem.a129148. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Batt R. D. Survival of Streptococcus lactis in starvation conditions. J Gen Microbiol. 1968 Mar;50(3):367–382. doi: 10.1099/00221287-50-3-367. [DOI] [PubMed] [Google Scholar]
- Thomas T. D., Jarvis B. D., Skipper N. A. Localization of proteinase(s) near the cell surface of Streptococcus lactis. J Bacteriol. 1974 May;118(2):329–333. doi: 10.1128/jb.118.2.329-333.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. I. Purification, stability, regulation of synthesis, and evidence for multiple molecular states. J Biol Chem. 1971 Mar 25;246(6):1733–1745. [PubMed] [Google Scholar]
- Tuominen F. W., Bernlohr R. W. Pyruvate kinase of the spore-forming bacterium, Bacillus licheniformis. II. Kinetic properties. J Biol Chem. 1971 Mar 25;246(6):1746–1755. [PubMed] [Google Scholar]


