Abstract
Deoxyribonucleic acid (DNA) and chemically defined media were used in transformation tests of 51 strains of Neisseria gonorrhoeae which exhibited various biosynthetic defects when isolated from patients. These auxotrophic gonococci had one or more nutritional requirements involving proline, methionine, arginine, hypoxanthine, uracil, and thiamine pyrophosphate (THPP). DNA from a clinical isolate which did not require these compounds for growth on defined medium transformed each of the auxotrophic markers of all 51 recipient populations. Ten isolates had defects involving the synthesis of THPP; four strains (designated Thp−) had a growth requirement that was satisfied only by THPP, whereas the requirement of six strains (designated Thi−) was satisfied by either thiamine or THPP. DNA from Thp− donors elicited transformation of Thp− as well as Thi− recipients. Reciprocally, DNA from a Thi− donor transformed both Thi− and Thp− recipients. Furthermore, DNA from other auxotrophic gonococci had transforming activity for some phenotypically similar auxotrophic recipients. The findings indicate the existence of various nonidentical genetic defects which block reactions in the biosynthesis of proline, methionine, arginine, hypoxanthine, and THPP. Routine cultures from patients with gonorrhea were the source of these auxotrophic strains of N. gonorrhoeae; the various nutritional requirements were identified by a recently described system of gonococcal auxotyping. The transformation test results verify the hereditary basis of the auxotypes, establish that many different mutations exist in potentially virulent gonococci, and illustrate the value of these auxotrophic mutants for studies of the genetic structure and evolution of natural populations of gonococci.
Full text
PDF


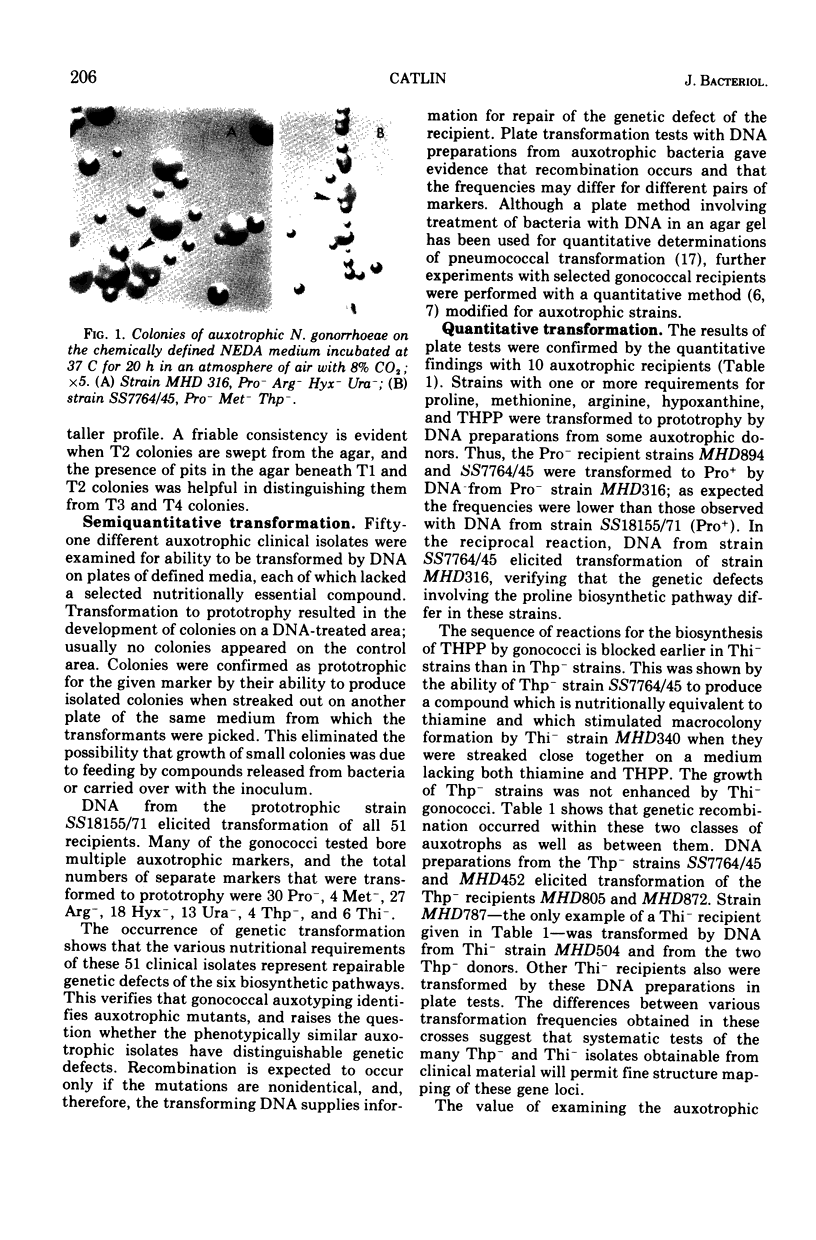
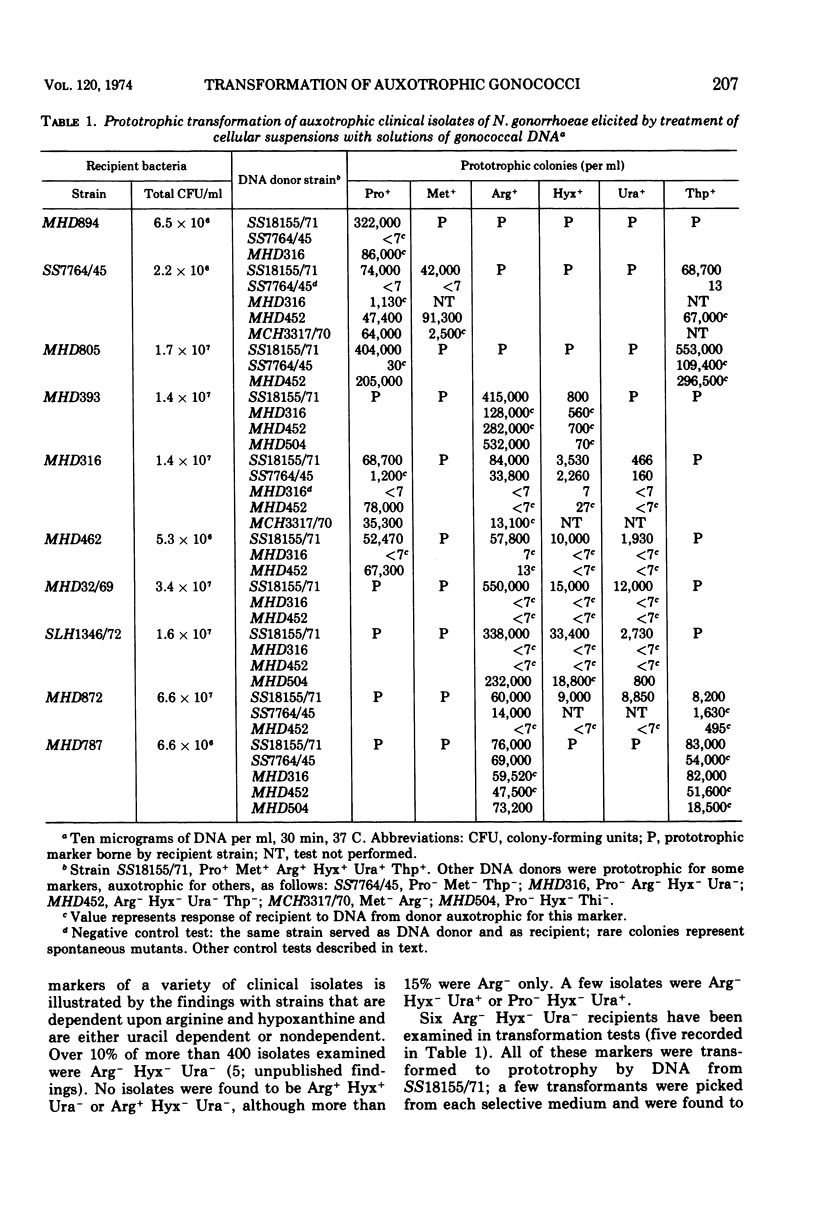


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- Buchanan T. M., Gotschlich E. C. Studies on gonococcus infection. 3. Correlation of gonococcal colony morphology with infectivity for the chick embryo. J Exp Med. 1973 Jan 1;137(1):196–200. doi: 10.1084/jem.137.1.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bumgarner L. R., Finkelstein R. A. Pathogenesis and immunology of experimental gonococcal infection: virulence of colony types of Neisseria gonorrhoeae for chicken embryos. Infect Immun. 1973 Dec;8(6):919–924. doi: 10.1128/iai.8.6.919-924.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CATLIN B. W. Transformation of Neisseria meningitidis by deoxyribonucleates from cells and from culture slime. J Bacteriol. 1960 Apr;79:579–590. doi: 10.1128/jb.79.4.579-590.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carifo K., Catlin B. W. Neisseria gonorrhoeae auxotyping: differentiation of clinical isolates based on growth responses on chemically defined media. Appl Microbiol. 1973 Sep;26(3):223–230. doi: 10.1128/am.26.3.223-230.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W., Bendler J. W., 3rd, Goodgal S. H. The type b capsulation locus of Haemophilus influenzae: map location and size. J Gen Microbiol. 1972 May;70(3):411–422. doi: 10.1099/00221287-70-3-411. [DOI] [PubMed] [Google Scholar]
- Catlin B. W. Genetic studies of sulfadiazine-resistant and methionine-requiring Neisseria isolated from clinical material. J Bacteriol. 1967 Sep;94(3):719–733. doi: 10.1128/jb.94.3.719-733.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Engelkirk P. G., Schoenhard D. E. Physical evidence of a plasmid in Neisseria gonorrhoeae. J Infect Dis. 1973 Feb;127(2):197–200. doi: 10.1093/infdis/127.2.197. [DOI] [PubMed] [Google Scholar]
- Fiumara N. J. The diagnosis and treatment of gonorrhea. Med Clin North Am. 1972 Sep;56(5):1105–1113. doi: 10.1016/s0025-7125(16)32336-7. [DOI] [PubMed] [Google Scholar]
- Jephcott A. E., Reyn A., Birch-Andersen A. Neisseria gonorrhoeae 3. Demonstration of presumed appendages to cells from different colony types. Acta Pathol Microbiol Scand B Microbiol Immunol. 1971;79(3):437–439. doi: 10.1111/j.1699-0463.1971.tb00086.x. [DOI] [PubMed] [Google Scholar]
- KELLOGG D. S., Jr, PEACOCK W. L., Jr, DEACON W. E., BROWN L., PIRKLE D. I. NEISSERIA GONORRHOEAE. I. VIRULENCE GENETICALLY LINKED TO CLONAL VARIATION. J Bacteriol. 1963 Jun;85:1274–1279. doi: 10.1128/jb.85.6.1274-1279.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellogg D. S., Jr, Cohen I. R., Norins L. C., Schroeter A. L., Reising G. Neisseria gonorrhoeae. II. Colonial variation and pathogenicity during 35 months in vitro. J Bacteriol. 1968 Sep;96(3):596–605. doi: 10.1128/jb.96.3.596-605.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Punsalang A. P., Jr, Sawyer W. D. Role of pili in the virulence of Neisseria gonorrhoeae. Infect Immun. 1973 Aug;8(2):255–263. doi: 10.1128/iai.8.2.255-263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RAVIN A. W. A quantitative study of autogenic and allogenic transformations in Pneumococcus. Exp Cell Res. 1954 Aug;7(1):58–82. doi: 10.1016/0014-4827(54)90042-7. [DOI] [PubMed] [Google Scholar]
- Sparling P. F. Antibiotic resistance in Neisseria gonorrhoeae. Med Clin North Am. 1972 Sep;56(5):1133–1144. doi: 10.1016/s0025-7125(16)32339-2. [DOI] [PubMed] [Google Scholar]
- Sparling P. F. Genetic transformation of Neisseria gonorrhoeae to streptomycin resistance. J Bacteriol. 1966 Nov;92(5):1364–1371. doi: 10.1128/jb.92.5.1364-1371.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J., Kraus S. J., Gotschlich E. C. Studies on gonococcus infection. I. Pili and zones of adhesion: their relation to gonococcal growth patterns. J Exp Med. 1971 Oct 1;134(4):886–906. doi: 10.1084/jem.134.4.886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. II. Freeze-fracture, freeze-etch studies on gonocci. J Exp Med. 1972 Nov 1;136(5):1258–1271. doi: 10.1084/jem.136.5.1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swanson J. Studies on gonococcus infection. IV. Pili: their role in attachment of gonococci to tissue culture cells. J Exp Med. 1973 Mar 1;137(3):571–589. doi: 10.1084/jem.137.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. W., Hill J. C., Tyeryar F. J., Jr Interaction of gonococci with phagocytic leukocytes from men and mice. Infect Immun. 1973 Jul;8(1):98–104. doi: 10.1128/iai.8.1.98-104.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thongthai C., Sawyer W. D. Studies on the virulence of Neisseria gonorrhoeae. I. Relation of colonial morphology and resistance to phagocytosis by polymorphonuclear leukocytes. Infect Immun. 1973 Mar;7(3):373–379. doi: 10.1128/iai.7.3.373-379.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHITE L. A., KELLOGG D. S., Jr NEISSERIA GONORRHOEAE IDENTIFICATION IN DIRECT SMEARS BY A FLUORESCENT ANTIBODY-COUNTERSTAIN METHOD. Appl Microbiol. 1965 Mar;13:171–174. doi: 10.1128/am.13.2.171-174.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willcox R. R. A world look at the venereal diseases: recrudescence of the venereal diseases. Med Clin North Am. 1972 Sep;56(5):1057–1071. doi: 10.1016/s0025-7125(16)32332-x. [DOI] [PubMed] [Google Scholar]




