Abstract
The relative abundance of alternatively spliced long (γ2L) and short (γ2S) mRNAs of the γ2 subunit of the γ-amino butyrate type A (GABAA) receptor was examined in dorsolateral prefrontal cortex of schizophrenics and matched controls by using in situ hybridization histochemistry and semiquantitative reverse transcription–PCR (RT-PCR) amplification. A cRNA probe identifying both mRNAs showed that the transcripts are normally expressed at moderately high levels in the prefrontal cortex. Consistent with previous studies, overall levels of γ2 transcripts in prefrontal cortex of brains from schizophrenics were reduced by 28.0%, although this reduction did not reach statistical significance. RT-PCR, performed under nonsaturating conditions on total RNA from the same blocks of tissue used for in situ hybridization histochemistry, revealed a marked reduction in the relative proportion of γ2S transcripts in schizophrenic brains compared with controls. In schizophrenics, γ2S transcripts had fallen to 51.7% (±7.9% SE; P < 0.0001) relative to control levels. Levels of γ2L transcripts showed only a small and nonsignificant reduction of 16.9% (±12.0% SE, P > 0.05). These findings indicate differential transcriptional regulation of two functionally distinct isoforms of one of the major GABAA receptor subunits in the prefrontal cortex of schizophrenics. The specific reduction in relative abundance of γ2S mRNAs and the associated relative increase in γ2L mRNAs should result in functionally less active GABAA receptors and have severe consequences for cortical integrative function.
Deficits of cognitive function in schizophrenia (1) reflect functional abnormality in interconnected prefrontal, temporal, and cingulate cortices (2). In the dorsolateral prefrontal cortex, functional hypoactivity (3) in schizophrenia is associated with defects in a number of neurotransmitter systems (4–11), including the inhibitory γ-amino butyrate (GABA)ergic system. Glutamic acid decarboxylase (GAD) mRNA levels are reduced in the prefrontal cortex of schizophrenics without loss of neurons (9). Release and uptake of GABA at synaptic terminals are reduced (12–14), and agonist binding to GABA type A (GABAA) receptors is altered. Muscimol shows increased binding in cingulate and prefrontal cortex of schizophrenics (15, 16), whereas other agonists, particularly benzodiazepines, a class of drugs commonly used in the treatment of schizophrenia, show decreased binding (17).
Benzodiazepine binding to GABAA receptors is modulated by the γ2 receptor subunit (18). γ2 subunits, along with α1 and β2 subunits, are the principal contributors to most native GABAA receptors (19–23), including in the primate cerebral cortex (24). α-Subunits are necessary for selectivity of the receptor for benzodiazepines (25), whereas the γ2 subunit is essential for high-affinity benzodiazepine binding (18, 25–31). γ2 subunits exist in two isoforms, products of alternative mRNA splicing: short (γ2S) and long (γ2L), the latter having an 8-aa insert (32, 33). Both isoforms may be important for benzodiazepine enhancement (30); γ2L encodes an additional phosphorylation site for protein kinase C (PKC; refs. 32 and 34) which, once phosphorylated, can alter channel function by negatively modulating GABA-activated currents (35). Varying levels of γ2S and γ2L occur in cerebral cortex (36–39).
Modest but statistically nonsignificant reductions of γ2 transcripts were found in prefrontal cortex of schizophrenics (40). This study did not, however, distinguish between the two alternatively spliced γ2 subunit mRNAs. In the present study, semiquantitative reverse transcription–PCR (RT-PCR) shows changes in relative levels of γ2S and γ2L mRNAs from prefrontal cortex of schizophrenics and matched controls.
MATERIALS AND METHODS
Material came from five pairs of adult brains. One brain from each pair was from a patient diagnosed with schizophrenia as defined by the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria; the other was from a control matched for age, gender, and autolysis time (the time between death and freezing of the brain; Table 1). All schizophrenics had a similar duration of illness and had been treated with conventional neuroleptics for a similar period of time. Subjects with a history of drug abuse or neurological illness or brains with evident Alzheimer pathology were excluded. Blocks, approximately 2 cm2 in size, were cut from the middle third of the superior frontal gyrus in frozen coronal slabs of each brain (41). Blocks were divided into two halves, one designated for histology and one for RNA extraction.
Table 1.
Characteristics of matched schizophrenic and control brain pairs
| Pair | Brain nos. | Diagnosis | Age, years | Sex | Autolysis time, hr |
|---|---|---|---|---|---|
| 1 | 2043 | Schizophrenic | 55 | M | 21.15 |
| 1901 | Control | 51 | M | 21.5 | |
| 2 | 2045 | Schizophrenic | 23 | M | 11 |
| 2168 | Control | 21 | M | 15 | |
| 3 | 2232 | Schizophrenic | 61 | F | 15 |
| 1756 | Control | 57 | F | 16.45 | |
| 4 | 2086 | Schizophrenic | 75 | F | 27.25 |
| 2065 | Control | 85 | F | 21.5 | |
| 5 | 2326 | Schizophrenic | 40 | M | 13.5 |
| 2338 | Control | 41 | M | 18 |
For in situ hybridization, each block was raised to 4°C over 20 min, fixed in cold 4% paraformaldehyde in 0.1 M phosphate buffer for ≈24 hr at 4°C and infiltrated with 30% sucrose, refrozen in dry ice, and sectioned serially at 40 μm on a freezing microtome. Free-floating sections were treated as described (40) and hybridized with 1 × 106 cpm/μl of [α-33P]UTP-labeled antisense (or sense) riboprobes transcribed from a linearized γ2 cDNA template (see below). Matched pairs were processed simultaneously. cRNA probe concentration and specific activity were approximately the same in each case.
After hybridization, the sections were exposed to β-max autoradiographic film (Amersham) for 10 days. After film development, slides were dipped in Kodak NTB2 photographic emulsion, exposed at 4°C for 6 weeks, developed in Kodak D19 developer, fixed with Kodak rapid fixer, and counterstained with cresyl violet acetate.
To quantify mRNA levels, optical density measurements were taken in repeated, nonoverlapping scans of defined width across the layers of the dorsolateral prefrontal cortex from pial surface into white matter 200 μm deep to layer VI of the cortex. Measurements, made on at least three sections from each brain, were converted to units of radioactivity per unit weight by reference to 14C standards (Amersham) exposed on the same sheet of film. All measurements were performed blind, and statistical significance was determined by unpaired Student’s t tests (statview 4.1, SAS Institute, Cary, NC) for the matched pairs, and by Kruskal–Wallis nonparametric ANOVA tests (instat 2.03; GraphPad, San Diego) for averaged results comparing schizophrenics and controls.
Subcloning and characterization of the monkey-specific cDNA template used for cRNA probe generation has been described (24). Synthetic oligonucleotide primers targeted to the cytoplasmic loop of the γ2 subunit were designed to flank a 477-bp region of the heterogeneous amino-terminal domain corresponding to bases 590–1,067 of the cDNA sequence of the γ2 subunit of the human GABAA receptor (25). Total RNA was isolated and reverse transcribed from monkey cerebral cortex, amplified by PCR, gel-fractionated, and subcloned into the pBS transcription vector (Stratagene). 33P-labeled antisense cRNA probes were generated by in vitro transcription of PvuII linearized templates using T3 RNA polymerase (Stratagene) and ethanol-precipitated. Sense probes for controls were generated by using T7 RNA polymerase.
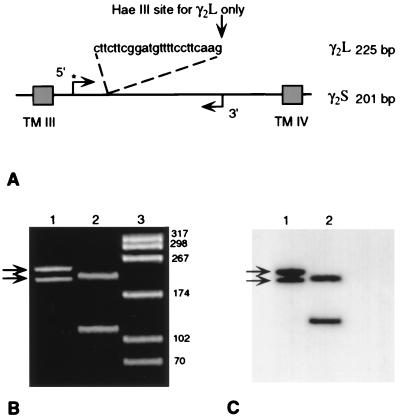
The second of the two frozen blocks from each brain was homogenized, and the total RNA was extracted by using the Molecular Research Center (Cincinnati) Tri Reagent single-step method (42). Aliquots of 50 ng of total RNA were converted to cDNA by using pd(N)6 random primers and transcribed with 100 units of Moloney murine leukemia virus reverse transcriptase (Pharmacia LKB) for first-strand cDNA synthesis. The forward primer for both γ2S and γ2L was 20 bp in length (5′-GCTCTGGTGGAGTATGGCAC-3′), and the reverse primer was 19 bp in length (5′-GGCACAGTCCTTGCCGTCC-3′); both oligonucleotide sequences were identical to the published human cDNA sequence (25) and spanned the 24-bp, alternatively spliced insert of γ2L (ref. 32; Fig. 1A). Primers for the endogenous mRNA, glyceraldehyde 3-phosphate dehydrogenase (G3PDH; CLONTECH), used as a standard for quantification were derived from exon 3 for the forward primer (5′-TATTGGGCGCCTGGTCACCA-3′) and were complementary to exon 4 for the reverse primer (5′-AGATGGTGATGGGATTTCCA-3′) yielding a 135-bp product. cDNAs, reverse transcribed from each schizophrenic and control pair, were subjected to 30 cycles of PCR amplification using 20 pmol of specific oligonucleotide primer pairs, 1× reaction buffers (GIBCO/BRL), 15 mM MgCl2, 200 μM mixed nucleotides (dNTPs), and 2.5 units of Taq DNA polymerase (GIBCO/BRL) in a Gene Amp 480 thermal cycler (Perkin–Elmer/Cetus). The forward primers for both the γ2 subunit and G3PDH cDNAs were labeled by inclusion of 20 units of T4 polynucleotide kinase (GIBCO/BRL) with ≈5 × 105 cpm of 32P-labeled forward primer. From all samples of prefrontal cortex RNA, both γ2S and γ2L transcripts were identified and characterized based on size and restriction mapping of products on 8% polyacrylamide gels after every 30 cycles of PCR amplification (Fig. 1 B and C). The resultant cDNAs were 201 and 225 bp in length, corresponded to γ2S and γ2L, respectively, and when digested with 10 units of HaeIII, the endogenous site created by the insertion at the 3′ end of γ2L was cut specifically (Fig. 1 B and C). In addition, individual products of γ2S and γ2L were isolated on an 8% agarose gel, electroeluted (Spectra-pore, Los Angeles), blunted with T4 DNA polymerase, and subcloned into the SmaI restriction site of Stratagene pBSII SK(+) with T4 DNA ligase and the SmaI restriction enzyme (Stratagene). After transformation into 71/18 competent cells (Stratagene) minipreps were gel-purified and sequenced by the dideoxy chain-termination method (43) by using the sequenase 2.0 version sequencing system (United States Biochemicals).
Figure 1.
Scheme illustrating primers used to amplify and to verify the identity of γ2S and γ2L mRNAs. (A) The amplified region of γ2S and γ2L occurred between the third and fourth transmembrane regions (TM III and TM IV), with the kinase-added 5′ forward primer indicated by ∗. (B) Ethidium bromide-stained polyacrylamide gel of amplified γ2S and γ2L cDNAs from a control brain at the predicted molecular weights of 201 and 225 bp (arrows in lane 1). Lane 2, same products digested with HaeIII, isolating the γ2L cDNA product. Lane 3, HaeIII-digested pBS to show molecular-weight markers. (C) Film autoradiogram of same gel showing the uncut, forward-primed 201- and 225-bp radiolabeled products in lane 1 (arrows) and forward-primed 201-bp fragment of γ2S (upper band) and the HaeIII-digested 108-bp fragment of γ2L in lane 2.
Relative mRNA abundance was determined from the relative amounts of radioactivity in each gel band by phosphorimager analysis using image-quant Version 3.0 (Molecular Dynamics) after exposure to autoradiographic film (Dupont/NEN) for 1 hr at room temperature.
RESULTS
Laminar Patterns of γ2 Transcripts.
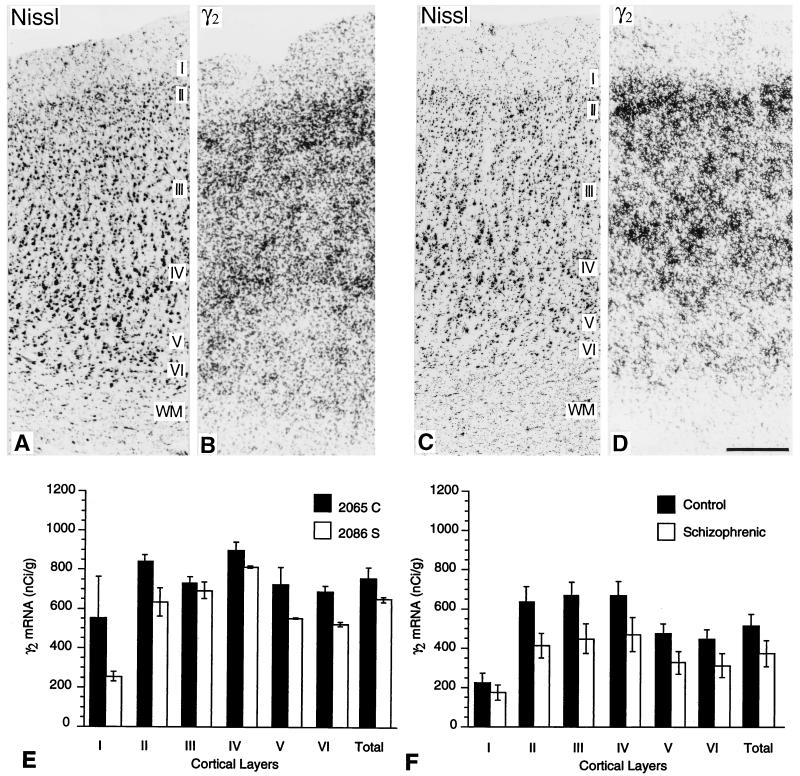
cRNA probes specific for γ2 subunit mRNA showed labeling of all six layers of the prefrontal cortex of controls and schizophrenics (Figs. 2 B and D) as described (40). Hybridization levels peaked in layer II, deep layer III, and layer IV. Labeling was weaker in layer VI and weakest in layers I and V (Fig. 2D). Sense controls revealed only nonspecific background labeling.
Figure 2.
(A–D) Photomicrographs from Nissl-stained sections (A and C) and from film autoradiograms (B and D) of adjacent sections through the dorsolateral prefrontal cortex of a control brain (A and B) and from a schizophrenic brain (C and D), hybridized with a radiolabeled γ2-specific cRNA probe. These show the pattern of distribution of γ2 mRNA transcripts across all six cortical layers. Sections from both schizophrenics and matched pairs were processed in the same hybridization medium and exposed for 10 days on the same sheet of film. (Bar = 0.3 mm.) (E and F) Histograms comparing levels of radiolabeled γ2 transcripts across cortical layers of the matched pair illustrated in A–D (E) and averages of transcript levels from the five pairs of brains examined (F). Data are shown as the mean of individual scans. Reductions in schizophrenics (open bars) in comparison with matched controls (shaded bars) do not reach statistical significance, except marginally in layers II and III.
Hybridization levels of γ2 transcripts were always lower in the prefrontal cortex of brains from schizophrenics (Fig. 2E). In prefrontal cortex of control brains, mean levels of mRNA for the γ2 subunit were 217.9 nCi/gm (1 Ci = 37 GBq) in layer I, 628.7 nCi/gm in layer II, 663.5 nCi/gm in layer III, 668.3 nCi/gm in layer IV, 469.9 nCi/gm in layer V, and 439.9 nCi/gm in layer VI. In prefrontal cortex of schizophrenic brains, mean levels were 171.9 nCi/gm in layer I, 409.2 nCi/gm in layer II, 444.3 nCi/gm in layer III, 466.7 nCi/gm in layer IV, 322.9 nCi/gm in layer V, and 309.0 nCi/gm in layer VI. In the schizophrenic cohort, there was a 21% decrease in layer I (P = 0.510), a 34% decrease in layer II (P = 0.046), a 33% decrease in layer III (P = 0.040), a 29% decrease in layer IV (P = 0.089), a 31% decrease in layer V (P = 0.080), and a 29% decrease in layer VI (P = 0.112). As indicated in parentheses, these differences reached a level of significance for layers II and III but did not reach significance for layers I, IV, V, or VI.
Relative Abundance of γ2S and γ2L mRNAs.
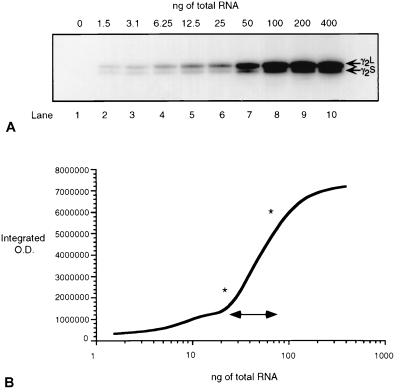
To quantify γ2S and γ2L transcript abundance, RT-PCR was performed under nonsaturating amplification conditions (Fig. 3). The linear range of amplification was determined in a 30-cycle RT-PCR amplification of reverse transcribed, randomly primed cDNAs, with increasing amounts (0–400 ng) of starting total RNA obtained from one schizophrenic brain (Fig. 3A). The linear range was estimated to lie between 25 and 100 ng of starting RNA (Fig. 3B), and all subsequent reactions were carried out by using 50 ng of starting RNA.
Figure 3.
Determination of linear range of amplification detectable with 30-cycle RT-PCR. (A) Serial dilutions (1:2) of total RNA were prepared and reverse-transcribed to generate γ2S and γ2L cDNA products, which were separated on an 8% polyacrylamide gel. Total RNA is shown in ng amounts above each lane, and the lane number is indicated below. (B) Phosphorimager quantification of vacuum-dried gel shown in A. ∗ and ↔ indicate the amount of starting RNA within the linear amplification range in which differences between schizophrenics and matched pairs should be accurately quantifiable.
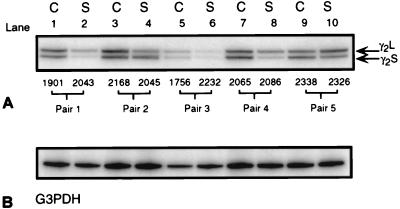
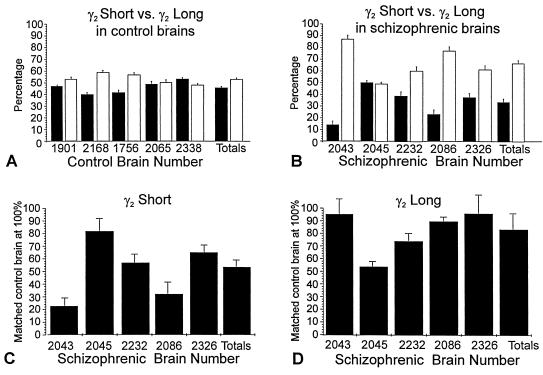
32P-end labeled primers targeted to the cytoplasmic loop of the γ2 subunit amplified both short and long (γ2S and γ2L) subunit mRNAs from both control and schizophrenic prefrontal cortex (Fig. 4A). Controls showed similar levels of amplified γ2S and γ2L mRNAs (Fig. 4A, odd-numbered lanes). γ2S and γ2L mRNA levels, as a percentage of total γ2 mRNA, were estimated by phosphorimager analysis (Fig. 5 A and B). In controls, γ2S and γ2L mRNAs were of approximately equal abundance. γ2L comprised 53.7% of the total mRNA content and γ2S 46.3% ± 1.28% (SE; all values reported are ±SE; P < 0.001).
Figure 4.
Digitized film autoradiograms of an 8% polyacrylamide gel from a typical RT-PCR experiment showing (A) amplified products from 50 ng of reverse transcribed total RNA from control (odd-numbered lanes) and schizophrenic cortex (even-numbered lanes). In every case, primers specifically amplified the 201-bp and 225-bp products γ2S and γ2L. Numbers above each pair are brain numbers for identification purposes. (B) PCR-amplified products for G3PDH from the same RT-reaction confirm first-strand synthesis efficiencies and enable semiquantification of expression levels.
Figure 5.
Histograms from phosphorimager analysis showing relative levels of γ2S and γ2L transcripts in dorsolateral prefrontal cortex of schizophrenics and control brains as quantified from five separate RT-PCR amplification experiments. (A and B) The abundance of γ2S mRNA (solid bars) compared as a percentage of total γ2 mRNA in control-brain cortical samples (A) and in schizophrenic-brain cortical samples (B). (C and D) The relative abundance of levels of γ2S (C) and γ2L (D) mRNAs normalized to G3PDH-amplified transcripts in all of the schizophrenic brain samples, compared with normalized levels obtained from their matched controls set at 100%.
By contrast, RT-PCR-amplified γ2S and γ2L mRNAs from schizophrenic prefrontal cortex showed a significant reduction in γ2S transcripts in all five brain samples (Fig. 4A, even-numbered lanes). On average, γ2S mRNAs from all schizophrenic brain samples constituted only 33.15% ± 3.19% (P < 0.0001) of total γ2 mRNA (Fig. 5B).
Abundance of γ2S and γ2L mRNAs.
Measurements of γ2S and γ2L mRNAs obtained from phosphorimager analysis were normalized to levels of G3PDH mRNA obtained from PCR amplification of G3PDH in the same reverse transcription reaction (Fig. 4). Transcription levels of G3PDH mRNA do not change in response to altered activity or to other inducing agents. All values for γ2S and γ2L mRNA amplified from schizophrenic prefrontal cortex were normalized to percentage levels of amplified G3PDH mRNAs obtained from the schizophrenic versus the control brain samples. These semiquantitative estimates (Fig. 5 C and D), the results of five separate RT-PCR amplification experiments, are shown as histograms of the percent difference of γ2S and γ2L levels in prefrontal cortex of each schizophrenic compared with levels obtained from the matched control, which was set at 100%. In all schizophrenic brain samples, levels of γ2S transcripts were reduced on average by 51.7% ± 7.9% (P < 0.001) compared with levels of γ2S transcripts in the matched controls (Fig. 5C). There was a small reduction (16.9% ± 12%) in γ2L mRNA in the same samples of prefrontal cortex from schizophrenics (Fig. 5D), but this was not significant (P > 0.05).
DISCUSSION
In a previous report on the dorsolateral prefrontal cortex of schizophrenics and matched controls (40), quantitative densitometry revealed consistently modest reductions in mRNA levels, including γ2 transcripts, in schizophrenic brains, but these changes did not reach statistical significance. In the present study, consistent with the previous findings, γ2 transcript expression was highest in layer II, deep layer III, and layer IV, with slightly lower levels in other layers. There was a modest, yet again nonsignificant, reduction of γ2 transcripts in four of the six layers of the same cortex in schizophrenics when compared with matched controls.
Using semiquantitative RT-PCR, total RNA isolated from the same tissue blocks used for in situ hybridization histochemistry was reverse transcribed and amplified using primers recognizing both γ2S and γ2L mRNAs. 32P-end labeled products were normalized to amplified levels of the ubiquitously expressed G3PDH. Overall, the percentage of γ2S mRNA was only slightly lower than γ2L in the control samples, with a γ2S/γ2L ratio of 46.3%:54.6% ± 1.28%(P < 0.001). In all of the schizophrenic brains, levels of γ2S mRNA were significantly reduced in comparison with γ2L mRNA (γ2S/γ2L ratio 33.15%:66.85% ± 3.19% in schizophrenics; P < 0.0001). There was a 51.7% ± 7.9% reduction (P < 0.001) of γ2S mRNA in schizophrenic brains versus matched controls, but only a slight overall reduction (16.9% ± 12.0%; P > 0.05) of γ2L mRNA, suggesting that the two alternatively spliced transcripts are differentially regulated in the prefrontal cortex in schizophrenia. A decrease in the nonspliced γ2S GABAA receptor subunit has been reported in the aging brain (39, 44), but this was not evident in the present study. Most of the controls, even the oldest, showed close to a 50:50 ratio of the short to long forms of γ2 mRNAs (Fig. 4A, brain 2065; Table 1).
Reduced γ2 Subunit Transcripts in Prefrontal Cortex of Schizophrenics May Reflect Reduced Inhibitory Capacity.
Decreases in γ2 subunit transcript levels of the kind reported here and marked decreases in benzodiazepine binding (17) indicate that a major component of the cortical inhibitory neurotransmitter system is affected in schizophrenics. Because γ2 subunits are essential for high-affinity benzodiazepine binding (22, 25–31, 45), especially for binding of flunitrazepam (18, 28), reduction in γ2 protein resulting from reduced transcript levels provides a basis for the decrease in 3H-flunitrazepam binding in schizophrenics.
The γ2 subunit is one of the most highly expressed and widely distributed GABAA receptor subunits in the mammalian central nervous system, including the developing and adult cerebral cortex (19–21, 24, 25, 46–49). It is normally colocalized with two other highly expressed subunits, α1 and β2 (20, 21, 24), suggesting that γ2, along with α1 and β2 subunits are fundamental components of the major subtype of GABAA receptors in the mammalian brain (19–21, 45, 47, 50). The pathophysiology of schizophrenia likely involves multiple defects of neurotransmitter function (11), including defects in the GABAergic system (51). In addition to alterations in receptor binding in schizophrenic cortex, there are also reductions in presynaptic turnover of GABA (12–14). Reduced GAD activity (14, 52) and reduced levels of GAD mRNA in schizophrenics without loss of neurons (9) indicates that down-regulation of GAD gene expression and thus of GABA production is an accompaniment of altered GABAA receptor function. Whether the defective GABAergic system is a primary element in the schizophrenic process or is secondary to other pathology, such as an altered pattern of cortical connections, remains unknown. However, many of the symptoms of schizophrenia may be attributable to alterations in the balance of intracortical excitation and inhibition.
Consequences of Differential Regulation of γ2S and γ2L Subunit Isoforms.
There was a considerable (51.7%) decrease in the abundance of γ2S mRNA in prefrontal cortex of schizophrenics. Because the accompanying reduction in γ2L mRNA was relatively minor, the alternatively spliced γ2L isoform is now the predominant γ2 GABAA receptor subunit in a compromised GABAergic transmitter system.
In the GABAergic system, phosphorylation of GABAA receptor subunits by a number of protein kinases is recognized as a potential mechanism for regulating inhibitory synaptic function (53, 54). For both γ2 subunits, phosphorylation by PKC occurs at Ser-327, but the γ2L isoform possesses an additional phosphorylation site at Ser-343 (34) within the 8-aa insert (32, 33). Phosphorylation negatively modulates GABAA receptors by reducing the amplitude of GABA-activated currents (35, 55). This reduction in GABA-mediated chloride currents is more severe for receptor subtypes containing more γ2L subunits relative to γ2S subunits because of the presence of the additional PKC site at Ser-343 (35). When coupled with already reduced γ2 mRNA levels, the overrepresentation of γ2L subunits should result in a functionally less active form of the GABAA receptor in the dorsolateral prefrontal cortex of schizophrenic brains.
The major changes detected in the present study are subtle, and the small decreases in overall γ2 mRNA would have been regarded as insignificant in the absence of knowledge of the changes in relative levels of γ2S and γ2L transcripts. There is a parallel effect on gene expression for N-methyl-d-aspartate receptor subunit mRNAs in the dorsolateral prefrontal cortex of schizophrenics: the NR2D subunit, which is normally expressed at low levels, is increased at the expense of other N-methyl-d-aspartate receptor transcripts, but without much change in overall transcript levels (10). The change was not found in brains from neuroleptic-treated nonschizophrenic controls. We have not yet confirmed that the change in γ2 subunit mRNAs is also unaffected by neuroleptic treatment, because all but one of the schizophrenic subjects in this study had been treated with neuroleptics. The change in NR2D receptor gene expression, like the change in relative proportions of γ2S and γ2L GABAA receptor subunits, should be accompanied by radically altered function at the N-methyl-d-aspartate receptor because recombinant receptors made up of NR1 and NR2D subunits are associated with prolonged decay times of glutamate-induced ion currents and a lower threshold for Mg2+ blockade in comparison with receptors assembled from NR1 and NR2A or NR2B subunits (56). The net effect should be to produce a receptor that is more “excitable” than normal, in the same cortex in which GABAA receptors are likely to be hypoactive. These are but two examples of changes in major cortical neurotransmitter systems in schizophrenia that should have profound consequences for cortical integrative function. The challenge is to determine to what extent these are primary defects or secondary, activity-dependent responses to a circuitry that is compromised by other underlying pathology.
Acknowledgments
We thank Dr. W. W. Tourtellotte and Ms. I. Rosario at the National Neurological Research Specimen Bank, Los Angeles, CA, for providing resources for storage and processing of brains; Dr. Dianne Hodges for helpful discussions; Mr. J. Beisner and the staff of the Orange County Sheriff/Coroner’s office; Dr. W. Lowell at the Ventura County Medical Examiner/Coroner’s office; and the families of the patients involved in this research. This work was supported by Grant MH54844 from the National Institutes of Health, United States Public Health Service (E.G.J.), by a Senior Investigator Award from the National Alliance for Research on Schizophrenia and Depression (E.G.J.), by a Stanley Award from the National Alliance for the Mentally Ill (E.G.J.) and by the Markey Foundation (M.M.H.).
ABBREVIATIONS
- GABA
γ-amino butyric acid
- GABAA
GABA type A receptor
- GAD
glutamic acid decarboxylase
- PKC
protein kinase C
- RT-PCR
reverse transcription–PCR
- γ2L
long form of the γ2 GABAA receptor subunit
- γ2S
short form of the γ2 GABAA receptor subunit
- G3PDH
glyceraldehyde 3-phosphate dehydrogenase
References
- 1.Liddle P P, Barnes T R E. Br J Psychiatry. 1990;157:558–561. doi: 10.1192/bjp.157.4.558. [DOI] [PubMed] [Google Scholar]
- 2.Weinberger D R, Berman K F, Suddath R, Torrey E F. Am J Psychiatry. 1992;149:890–897. doi: 10.1176/ajp.149.7.890. [DOI] [PubMed] [Google Scholar]
- 3.Winn P. Trends Neurosci. 1994;17:265–268. doi: 10.1016/0166-2236(94)90054-x. [DOI] [PubMed] [Google Scholar]
- 4.Grace A A. Neuroscience. 1991;41:1–24. doi: 10.1016/0306-4522(91)90196-u. [DOI] [PubMed] [Google Scholar]
- 5.Schmauss C, Haroutunian V, Davis K L, Davidson M. Proc Natl Acad Sci USA. 1993;90:8942–8946. doi: 10.1073/pnas.90.19.8942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Seeman P, Guan H-C, Van Tol H H M. Nature (London) 1993;365:441–444. doi: 10.1038/365441a0. [DOI] [PubMed] [Google Scholar]
- 7.Bunney B G, Bunney W E, Jr, Carlsson A. In: Psychopharmacology, the Fourth Generation of Progress. Bloom F E, Kupfer D J, editors. New York: Raven; 1995. pp. 1205–1214. [Google Scholar]
- 8.Olney J W, Farber N B. Arch Gen Psychiatry. 1995;52:998–1007. doi: 10.1001/archpsyc.1995.03950240016004. [DOI] [PubMed] [Google Scholar]
- 9.Akbarian S, Kim J J, Potkin S G, Hagman J O, Tafazzoli A, Bunney W E, Jr, Jones E G. Arch Gen Psychiatry. 1995;2:258–266. doi: 10.1001/archpsyc.1995.03950160008002. [DOI] [PubMed] [Google Scholar]
- 10.Akbarian S, Sucher N J, Bradley D, Tafazzoli A, Trinh D, Hetrick W P, Potkin S G, Sandman C A, Bunney W E, Jr, Jones E G. J Neurosci. 1996;16:19–30. doi: 10.1523/JNEUROSCI.16-01-00019.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nestler E J. Nature (London) 1997;385:578–579. doi: 10.1038/385578a0. [DOI] [PubMed] [Google Scholar]
- 12.Simpson M D, Slater P, Deakin J F, Royston M C, Skan W J. Neurosci Lett. 1989;107:211–215. doi: 10.1016/0304-3940(89)90819-7. [DOI] [PubMed] [Google Scholar]
- 13.Reynolds G P, Czudek C, Andrews H B. Biol Psychiatry. 1990;27:1038–1044. doi: 10.1016/0006-3223(90)90039-5. [DOI] [PubMed] [Google Scholar]
- 14.Sherman A D, Davidson A T, Baruah S, Hegwood T S, Waziri R. Neurosci Lett. 1991;121:77–80. doi: 10.1016/0304-3940(91)90653-b. [DOI] [PubMed] [Google Scholar]
- 15.Benes F M, Vincent S L, Alsterberg G, Bird E D, San Giovani J P. J Neurosci. 1992;12:924–929. doi: 10.1523/JNEUROSCI.12-03-00924.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benes F M, Vincent S L, Marie A, Khan Y. Neuroscience. 1996;75:1021–1031. doi: 10.1016/0306-4522(96)00328-4. [DOI] [PubMed] [Google Scholar]
- 17.Squires R F, Lajtha A, Saederup E, Palkovits M. Neurochem Res. 1993;18:219–223. doi: 10.1007/BF01474687. [DOI] [PubMed] [Google Scholar]
- 18.Wafford K A, Bain C J, Whiting P J, Kemp J A. Mol Pharmacol. 1993;44:437–442. [PubMed] [Google Scholar]
- 19.Gambarana C, Beattie C E, Rodriguez Z R, Seigel R E. Neuroscience. 1991;45:423–432. doi: 10.1016/0306-4522(91)90238-j. [DOI] [PubMed] [Google Scholar]
- 20.Persohn E, Malherbe P, Richards J G. J Comp Neurol. 1992;326:193–216. doi: 10.1002/cne.903260204. [DOI] [PubMed] [Google Scholar]
- 21.Wisden W, Laurie D J, Monyer H, Seeburg P H. J Neurosci. 1992;12:1040–1062. doi: 10.1523/JNEUROSCI.12-03-01040.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sigel R E, Baur R, Trube G, Möhler H, Malherbe P. Neuron. 1990;5:703–711. doi: 10.1016/0896-6273(90)90224-4. [DOI] [PubMed] [Google Scholar]
- 23.Verdoorn T A, Draguhn A, Ymer S, Seeburg P H, Sakmann B. Neuron. 1990;4:919–928. doi: 10.1016/0896-6273(90)90145-6. [DOI] [PubMed] [Google Scholar]
- 24.Huntsman M M, Isackson P J, Jones E G. J Neurosci. 1994;14:2236–2259. doi: 10.1523/JNEUROSCI.14-04-02236.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pritchett D B, Sontheimer H, Shivers B D, Ymer S, Kettenmann H, Schofield P R, Seeburg P H. Nature (London) 1989;338:582–585. doi: 10.1038/338582a0. [DOI] [PubMed] [Google Scholar]
- 26.Pritchett D B, Lüddens H, Seeburg P H. Science. 1989;245:1389–1392. doi: 10.1126/science.2551039. [DOI] [PubMed] [Google Scholar]
- 27.Pritchett D B, Seeburg P H. J Neurochem. 1990;54:1802–1804. doi: 10.1111/j.1471-4159.1990.tb01237.x. [DOI] [PubMed] [Google Scholar]
- 28.von Blankenfeld G, Ymer S, Pritchett D B, Sontheimer H, Ewert M, Seeburg P H, Kettenmann H. Neurosci Lett. 1990;115:269–273. doi: 10.1016/0304-3940(90)90467-n. [DOI] [PubMed] [Google Scholar]
- 29.Knoflach F, Rhyner T, Villa M, Kellenberger S, Drescher U, Malherbe P, Sigel E, Mohler H. FEBS Lett. 1991;293:191–194. doi: 10.1016/0014-5793(91)81184-a. [DOI] [PubMed] [Google Scholar]
- 30.Wafford K A, Burnett D M, Leidenheimer N J, Burt D R, Wang J B, Kofuji P, Dunwiddie T V, Harris R A, Sikela J M. Neuron. 1991;7:27–33. doi: 10.1016/0896-6273(91)90071-7. [DOI] [PubMed] [Google Scholar]
- 31.Angelotti T P, MacDonald R L. J Neurosci. 1993;13:1429–1440. doi: 10.1523/JNEUROSCI.13-04-01429.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Whiting P, McKernan R M, Iversen L L. Proc Natl Acad Sci USA. 1990;87:9966–9970. doi: 10.1073/pnas.87.24.9966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kofuji P, Wang J B, Moss S J, Huganir R L, Burt D R. J Neurochem. 1991;56:713–715. doi: 10.1111/j.1471-4159.1991.tb08209.x. [DOI] [PubMed] [Google Scholar]
- 34.Moss S J, Doherty C A, Huganir R L. J Biol Chem. 1992;267:14470–14476. [PubMed] [Google Scholar]
- 35.Krishek B J, Xie X, Blackstone C, Huganir R L, Moss S J, Smart T G. Neuron. 1994;12:1081–1095. doi: 10.1016/0896-6273(94)90316-6. [DOI] [PubMed] [Google Scholar]
- 36.Bovolin P, Santi M-R, Memo M, Costa E, Grayson D R. J Neurochem. 1992;59:62–72. doi: 10.1111/j.1471-4159.1992.tb08876.x. [DOI] [PubMed] [Google Scholar]
- 37.Khan Z U, Gutiérrez A, De Blas A L. J Neurochem. 1994;63:1466–1476. doi: 10.1046/j.1471-4159.1994.63041466.x. [DOI] [PubMed] [Google Scholar]
- 38.Miralles C P, Gutiérrez A, Khan Z U, Vitorica J, De Blas A L. Mol Brain Res. 1994;24:129–139. doi: 10.1016/0169-328x(94)90124-4. [DOI] [PubMed] [Google Scholar]
- 39.Gutiérrez A, Khan Z U, Miralles C P, De Blas A L. Mol Brain Res. 1996;35:91–102. doi: 10.1016/0169-328x(95)00187-w. [DOI] [PubMed] [Google Scholar]
- 40.Akbarian S, Huntsman M M, Kim J J, Tafazzoli A, Potkin S G, Bunney W E, Jr, Jones E G. Cereb Cortex. 1995;5:550–560. doi: 10.1093/cercor/5.6.550. [DOI] [PubMed] [Google Scholar]
- 41.Jones E G, Hendry S H C, Liu X-B, Potkin S G, Tourtellotte W W. J Neurosci Methods. 1992;44:133–144. doi: 10.1016/0165-0270(92)90006-y. [DOI] [PubMed] [Google Scholar]
- 42.Chomczynski P. BioTechniques. 1993;15:532–537. [PubMed] [Google Scholar]
- 43.Sanger F, Nicklin S, Coulson A R. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gutiérrez A, Khan Z U, Morris S J, De Blas A L. J Neurosci. 1994;14:7469–7477. doi: 10.1523/JNEUROSCI.14-12-07469.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stephenson F A, Duggan M J, Pollard S. J Biol Chem. 1990;265:21160–21165. [PubMed] [Google Scholar]
- 46.Shivers B D, Killisch I, Sprengel R, Sontheimer H, Kohler M, Schofield P R, Seeburg P H. Neuron. 1989;3:327–337. doi: 10.1016/0896-6273(89)90257-2. [DOI] [PubMed] [Google Scholar]
- 47.Laurie D J, Seeburg P H, Wisden W. J Neurosci. 1992;12:4151–4172. doi: 10.1523/JNEUROSCI.12-11-04151.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Huntsman M M, Leggio M G, Jones E G. J Neurosci. 1996;16:3571–3589. doi: 10.1523/JNEUROSCI.16-11-03571.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Golshani P, Truong H, Jones E G. J Comp Neurol. 1997;383:199–219. doi: 10.1002/(sici)1096-9861(19970630)383:2<199::aid-cne7>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 50.Benke D, Mertens S, Trzeciake A, Gillessen D, Mohler H. J Biol Chem. 1991;266:4478–4483. [PubMed] [Google Scholar]
- 51.Lee D E, Tobin A J. Arch Gen Psychiatry. 1995;52:267–268. doi: 10.1001/archpsyc.1995.03950160017003. [DOI] [PubMed] [Google Scholar]
- 52.Bird E D, Spokes E G S, Iversen L L. Brain. 1979;102:347–360. doi: 10.1093/brain/102.2.347. [DOI] [PubMed] [Google Scholar]
- 53.Leidenheimer N J, Browning M D, Harris R A. Trends Pharmacol Sci. 1991;12:84–87. doi: 10.1016/0165-6147(91)90509-q. [DOI] [PubMed] [Google Scholar]
- 54.Swope S L, Moss S J, Blackstone C D, Huganir R L. FASEB J. 1992;6:2514–2523. [PubMed] [Google Scholar]
- 55.Kellenberger S, Malherbe P, Sigel E. J Biol Chem. 1992;267:25660–25663. [PubMed] [Google Scholar]
- 56.Monyer H, Burnashev N, Laurie D J, Sakmann B, Seeburg P H. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]