Abstract
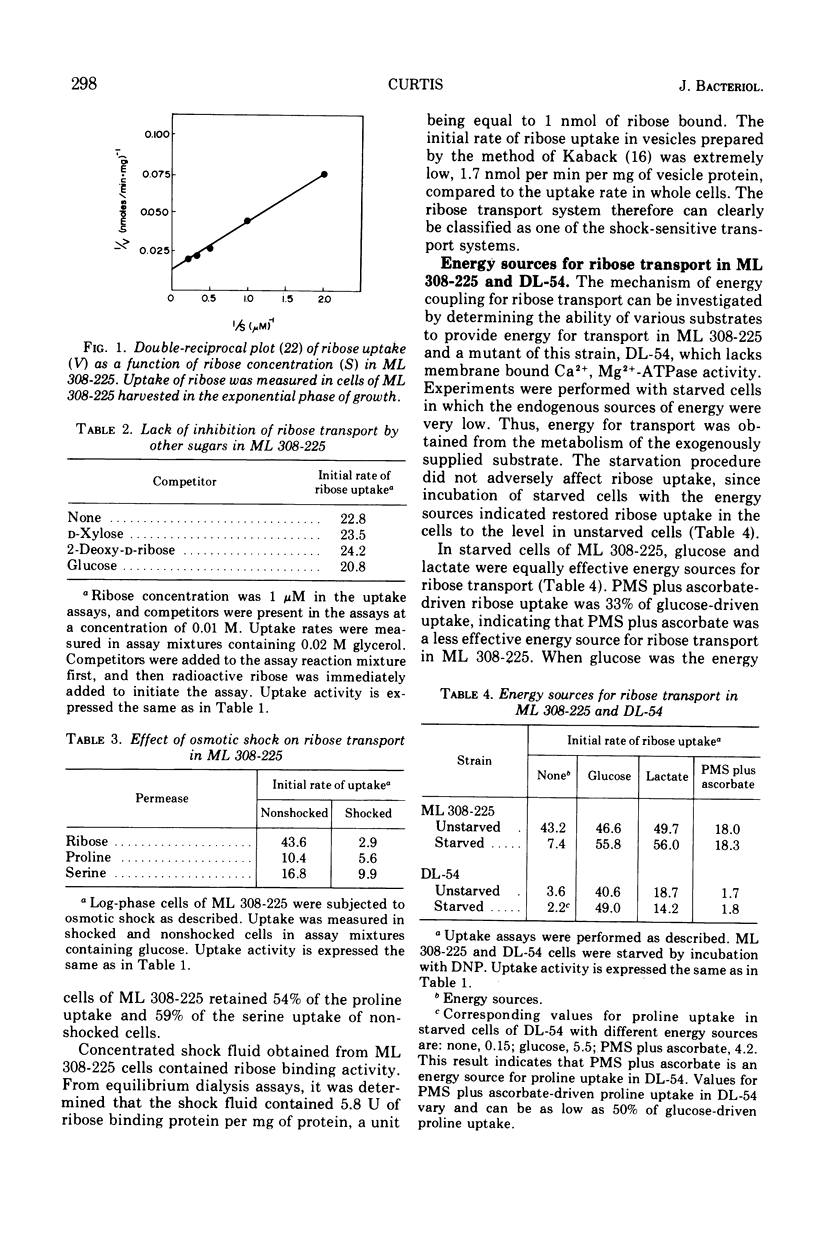
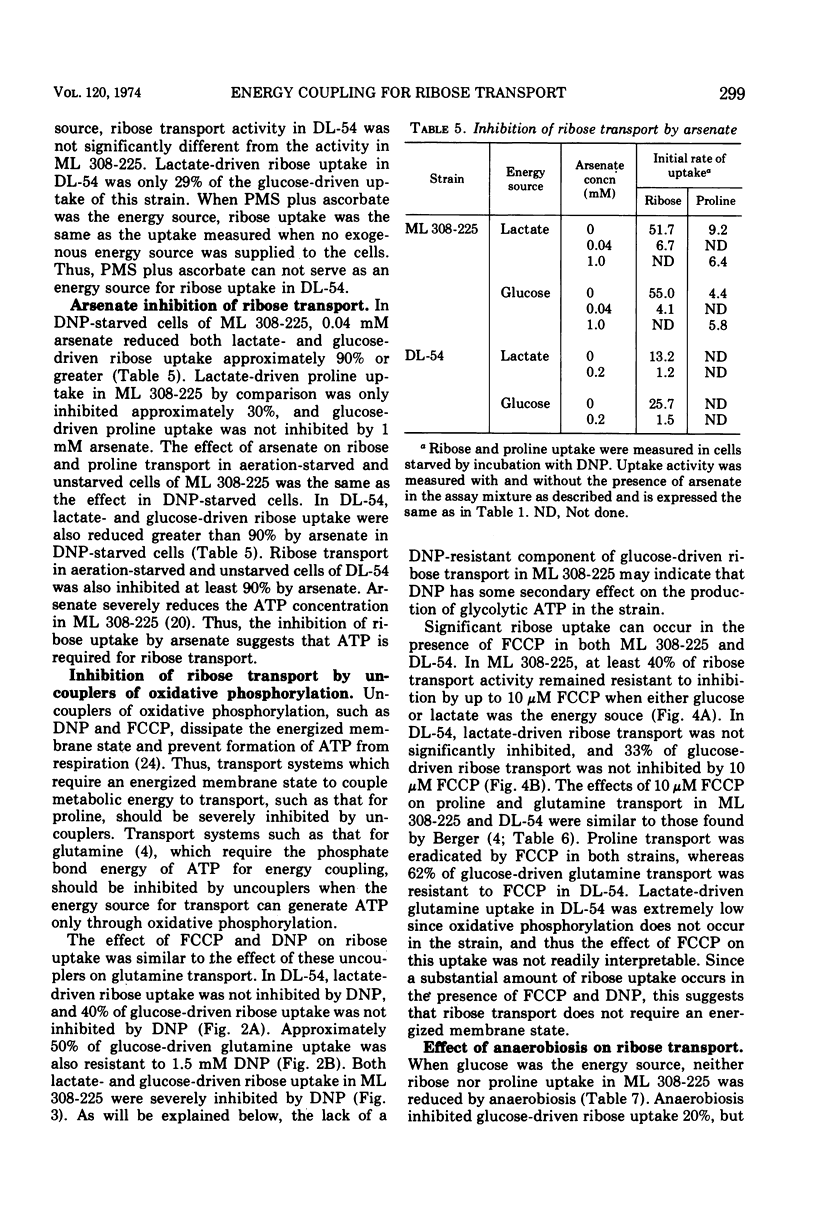
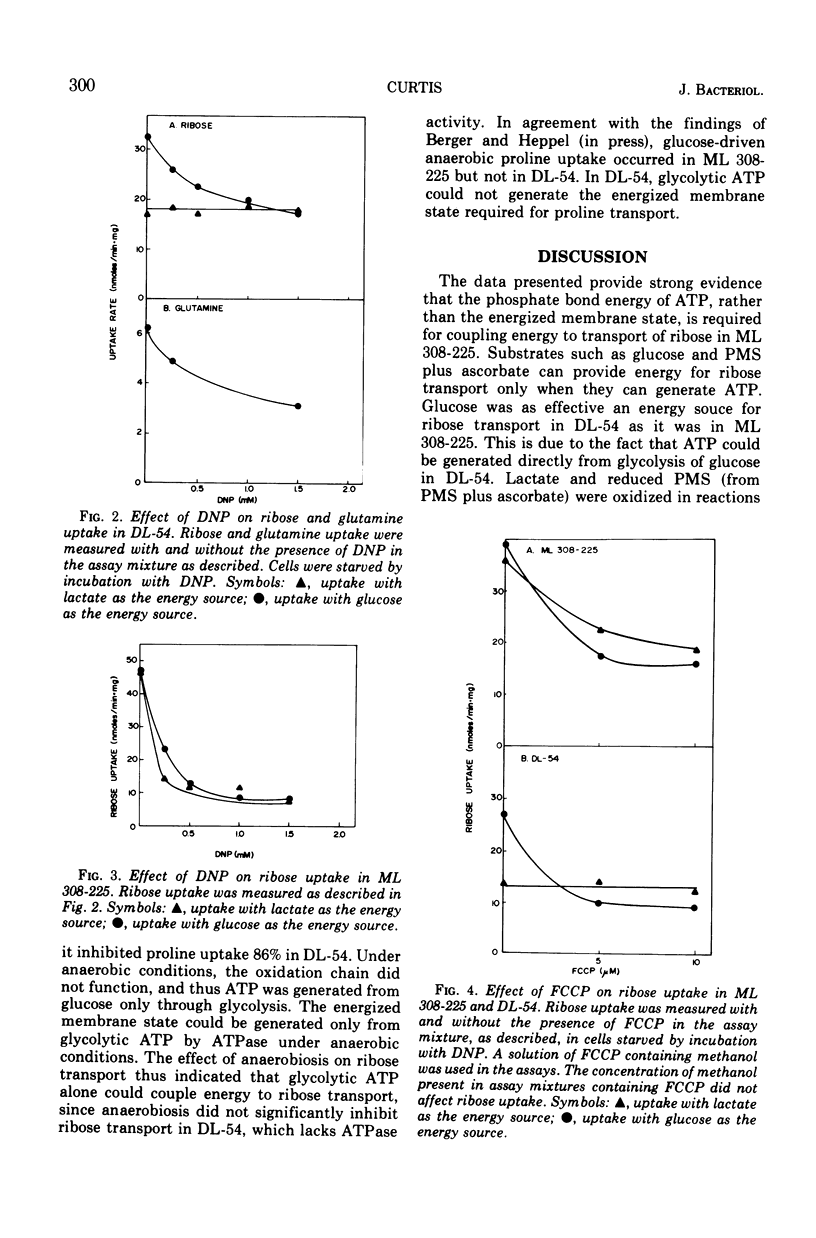
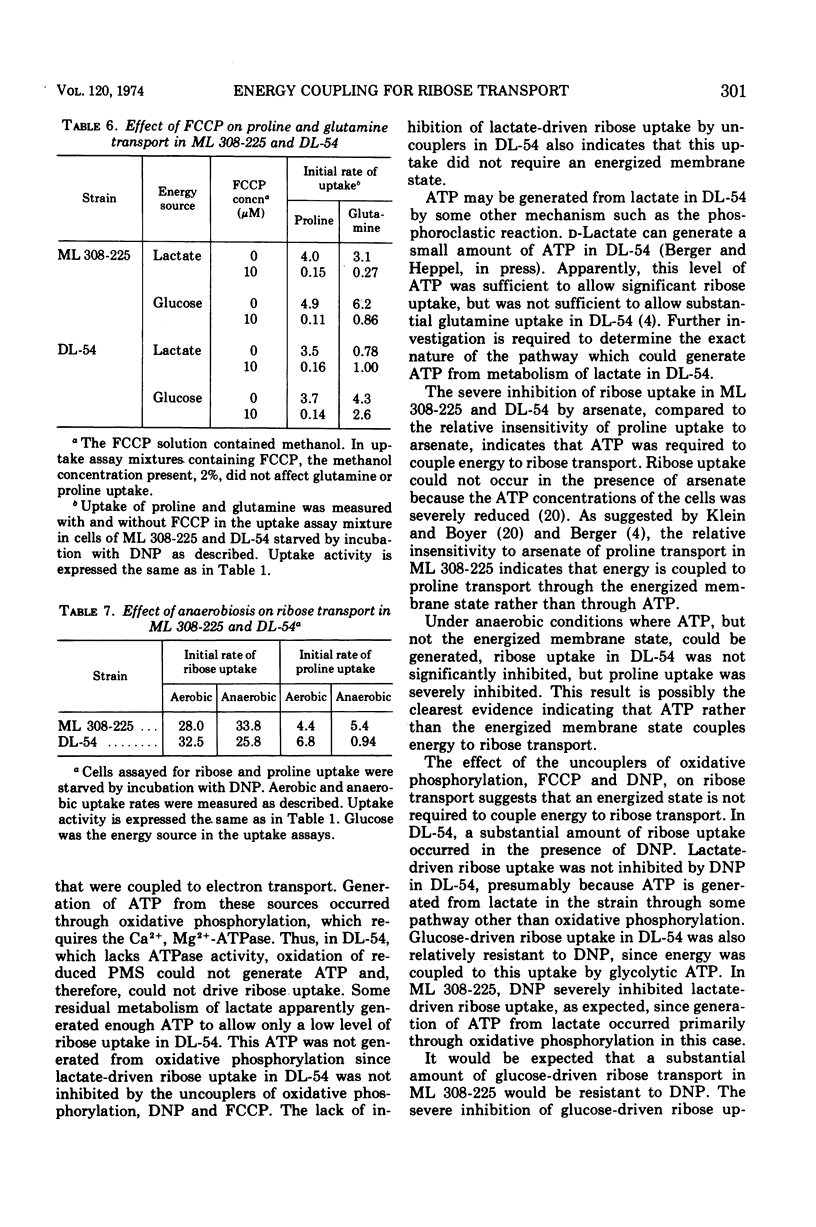
In Escherichia coli ML 308-225, d-ribose is transported into the cell by a constitutive active transport system of high activity. The activity of this transport system is severely reduced in cells subjected to osmotic shock, and the system is not present in membrane vesicles. The mechanism by which metabolic energy is coupled to transport of ribose was investigated. Substrates which generate adenosine 5′-triphosphate primarily through oxidative phosphorylation are poor energy sources for ribose uptake in DL-54, a mutant of ML 308-225 which lacks activity for the membrane-bound Ca2+, Mg2+-dependent adenosine triphosphatase required for oxidative phosphorylation. Arsenate severely inhibits ribose uptake, whereas, under the same conditions, uptake of l-proline is relatively insensitive to arsenate. Anaerobiosis does not significantly inhibit ribose uptake in ML 308-225 or DL-54 when glucose is the energy source. A significant amount of ribose uptake is resistant to uncouplers of oxidative phosphorylation such as 2,4-dinitrophenol. These results indicate that the phosphate bond energy of adenosine 5′-triphosphate, rather than an energized membrane state, couples energy to ribose transport in ML 308-225.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abou-Sabé M., Richman J. On the regulation of D-ribose metabolism in E. coli B-r. I. Isolation and characterization of D-ribokinaseless and D-ribose permeaseless mutants. Mol Gen Genet. 1973 May 28;122(4):291–301. doi: 10.1007/BF00269429. [DOI] [PubMed] [Google Scholar]
- Aksamit R., Koshland D. E., Jr A ribose binding protein of Salmonella typhimurium. Biochem Biophys Res Commun. 1972 Sep 26;48(6):1348–1353. doi: 10.1016/0006-291x(72)90860-1. [DOI] [PubMed] [Google Scholar]
- Anderson A., Cooper R. A. Biochemical and genetical studies on ribose catabolism in Escherichia coli K12. J Gen Microbiol. 1970 Aug;62(3):335–339. doi: 10.1099/00221287-62-3-335. [DOI] [PubMed] [Google Scholar]
- Berger E. A. Different mechanisms of energy coupling for the active transport of proline and glutamine in Escherichia coli. Proc Natl Acad Sci U S A. 1973 May;70(5):1514–1518. doi: 10.1073/pnas.70.5.1514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragg P. D., Hou C. Reconstitution of energy-dependent transhydrogenase in ATPase-negative mutants of Escherichia coli. Biochem Biophys Res Commun. 1973 Feb 5;50(3):729–736. doi: 10.1016/0006-291x(73)91305-3. [DOI] [PubMed] [Google Scholar]
- Butlin J. D., Cox G. B., Gibson F. Oxidative phosphorylation in Escherichia coli K12. Mutations affecting magnesium ion- or calcium ion-stimulated adenosine triphosphatase. Biochem J. 1971 Aug;124(1):75–81. doi: 10.1042/bj1240075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox G. B., Newton N. A., Butlin J. D., Gibson F. The energy-linked transhydrogenase reaction in respiratory mutants of Escherichia coli K12. Biochem J. 1971 Nov;125(2):489–493. doi: 10.1042/bj1250489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David J., Wiesmeyer H. Regulation of ribose metabolism in Escherichia coli. I. The ribose catabolic pathway. Biochim Biophys Acta. 1970 Apr 14;208(1):45–55. doi: 10.1016/0304-4165(70)90047-4. [DOI] [PubMed] [Google Scholar]
- EGGLESTON L. V., KREBS H. A. Permeability of Escherichia coli to ribose and ribose nucleotides. Biochem J. 1959 Oct;73:264–270. doi: 10.1042/bj0730264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox C. F., Kennedy E. P. Specific labeling and partial purification of the M protein, a component of the beta-galactoside transport system of Escherichia coli. Proc Natl Acad Sci U S A. 1965 Sep;54(3):891–899. doi: 10.1073/pnas.54.3.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furlong C. E., Morris R. G., Kandrach M., Rosen B. P. A multichamber equilibrium dialysis apparatus. Anal Biochem. 1972 Jun;47(2):514–526. doi: 10.1016/0003-2697(72)90146-7. [DOI] [PubMed] [Google Scholar]
- Harold F. M. Conservation and transformation of energy by bacterial membranes. Bacteriol Rev. 1972 Jun;36(2):172–230. doi: 10.1128/br.36.2.172-230.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbauer G. L., Adler J. Role of the galactose binding protein in chemotaxis of Escherichia coli toward galactose. Nat New Biol. 1971 Mar 24;230(12):101–104. doi: 10.1038/newbio230101a0. [DOI] [PubMed] [Google Scholar]
- Hong J. S., Kaback H. R. Mutants of Salmonella typhimurium and Escherichia coli pleiotropically defective in active transport. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3336–3340. doi: 10.1073/pnas.69.11.3336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaback H. R. Transport across isolated bacterial cytoplasmic membranes. Biochim Biophys Acta. 1972 Aug 4;265(3):367–416. doi: 10.1016/0304-4157(72)90014-7. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Energy linked nicotinamide adenine dinucleotide transhydrogenase in a mutant of Escherichia coli K12 lacking membrane Mg(2+)&z.sbnd;Ca(2+)-activated adenosine triphosphatase. FEBS Lett. 1972 May 1;22(2):197–199. doi: 10.1016/0014-5793(72)80043-7. [DOI] [PubMed] [Google Scholar]
- Kanner B. I., Gutnick D. L. Use of neomycin in the isolation of mutants blocked in energy conservation in Escherichia coli. J Bacteriol. 1972 Jul;111(1):287–289. doi: 10.1128/jb.111.1.287-289.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein W. L., Boyer P. D. Energization of active transport by Escherichia coli. J Biol Chem. 1972 Nov 25;247(22):7257–7265. [PubMed] [Google Scholar]
- LARDY H. A., WELLMAN H. Oxidative phosphorylations; rôle of inorganic phosphate and acceptor systems in control of metabolic rates. J Biol Chem. 1952 Mar;195(1):215–224. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Neu H. C., Heppel L. A. The release of enzymes from Escherichia coli by osmotic shock and during the formation of spheroplasts. J Biol Chem. 1965 Sep;240(9):3685–3692. [PubMed] [Google Scholar]
- Pavlasova E., Harold F. M. Energy coupling in the transport of beta-galactosides by Escherichia coli: effect of proton conductors. J Bacteriol. 1969 Apr;98(1):198–204. doi: 10.1128/jb.98.1.198-204.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prezioso G., Hong J. S., Kerwar G. K., Kaback H. R. Mechanisms of active transport in isolated bacterial membrane vesicles. XII. Active transport by a mutant of Escherichia coli uncoupled for oxidative phosphorylation. Arch Biochem Biophys. 1973 Feb;154(2):575–582. doi: 10.1016/0003-9861(73)90011-8. [DOI] [PubMed] [Google Scholar]
- Schairer H. U., Haddock B. A. -Galactoside accumulation in a Mg 2+ -,Ca 2+ -activated ATPase deficient mutant of E.coli. Biochem Biophys Res Commun. 1972 Aug 7;48(3):544–551. doi: 10.1016/0006-291x(72)90382-8. [DOI] [PubMed] [Google Scholar]
- Simoni R. D., Shallenberger M. K. Coupling of energy to active transport of amino acids in Escherichia coli. Proc Natl Acad Sci U S A. 1972 Sep;69(9):2663–2667. doi: 10.1073/pnas.69.9.2663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Lerner S. A., Lin E. C. Replacement of a phosphoenolpyruvate-dependent phosphotransferase by a nicotinamide adenine dinucleotide-linked dehydrogenase for the utilization of mannitol. J Bacteriol. 1967 Feb;93(2):642–648. doi: 10.1128/jb.93.2.642-648.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]



