Abstract
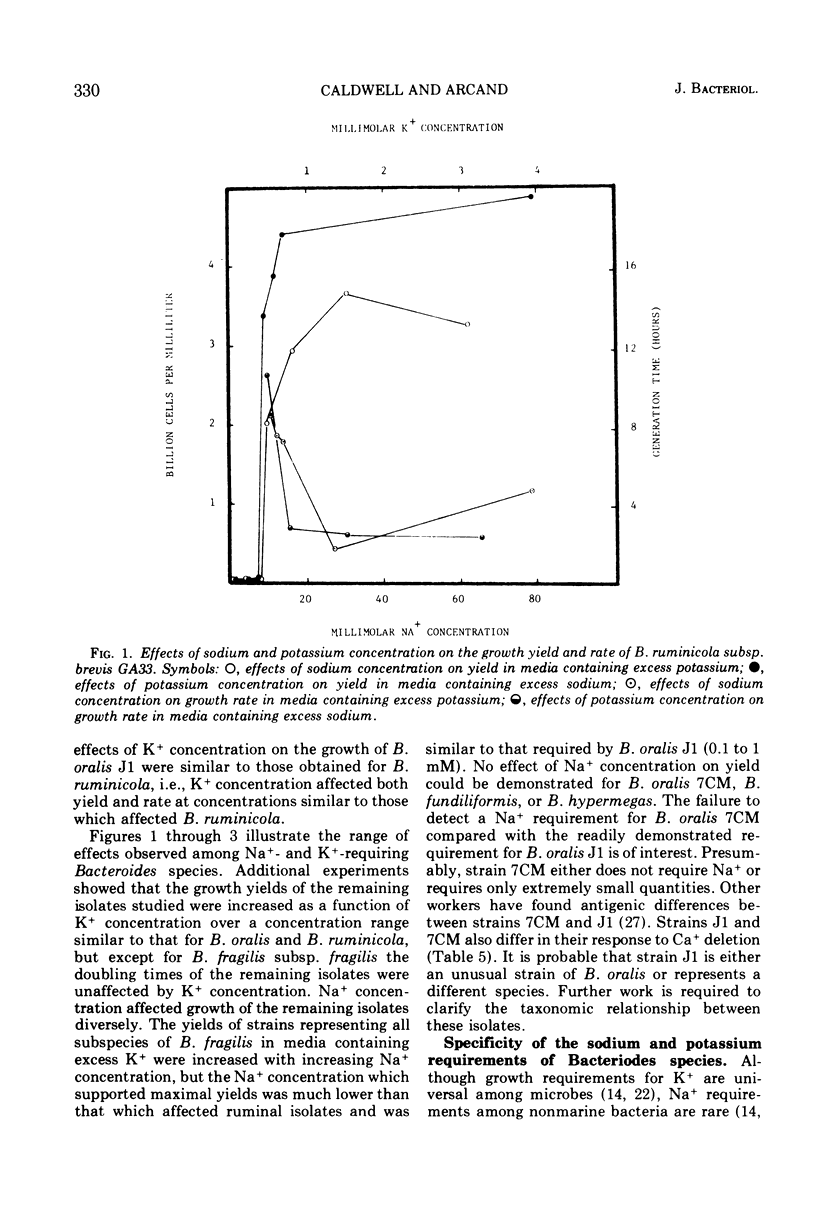
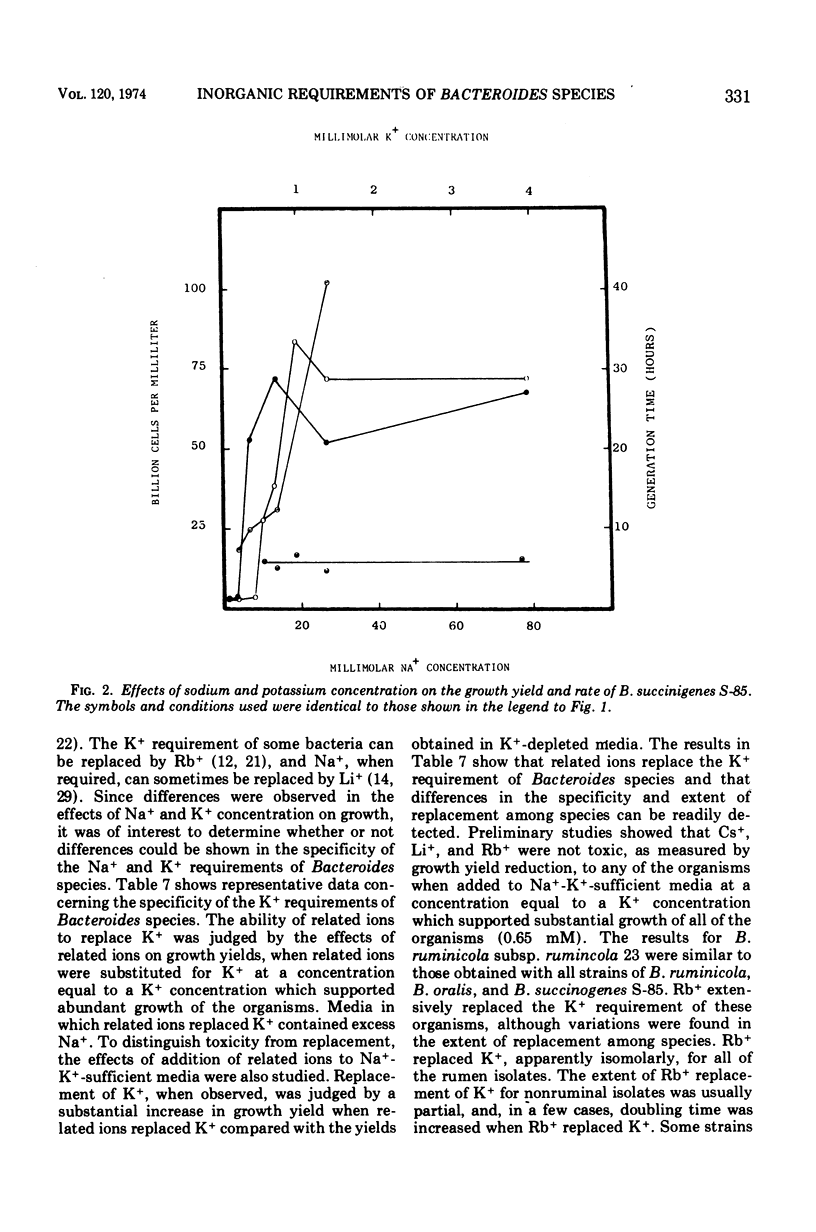
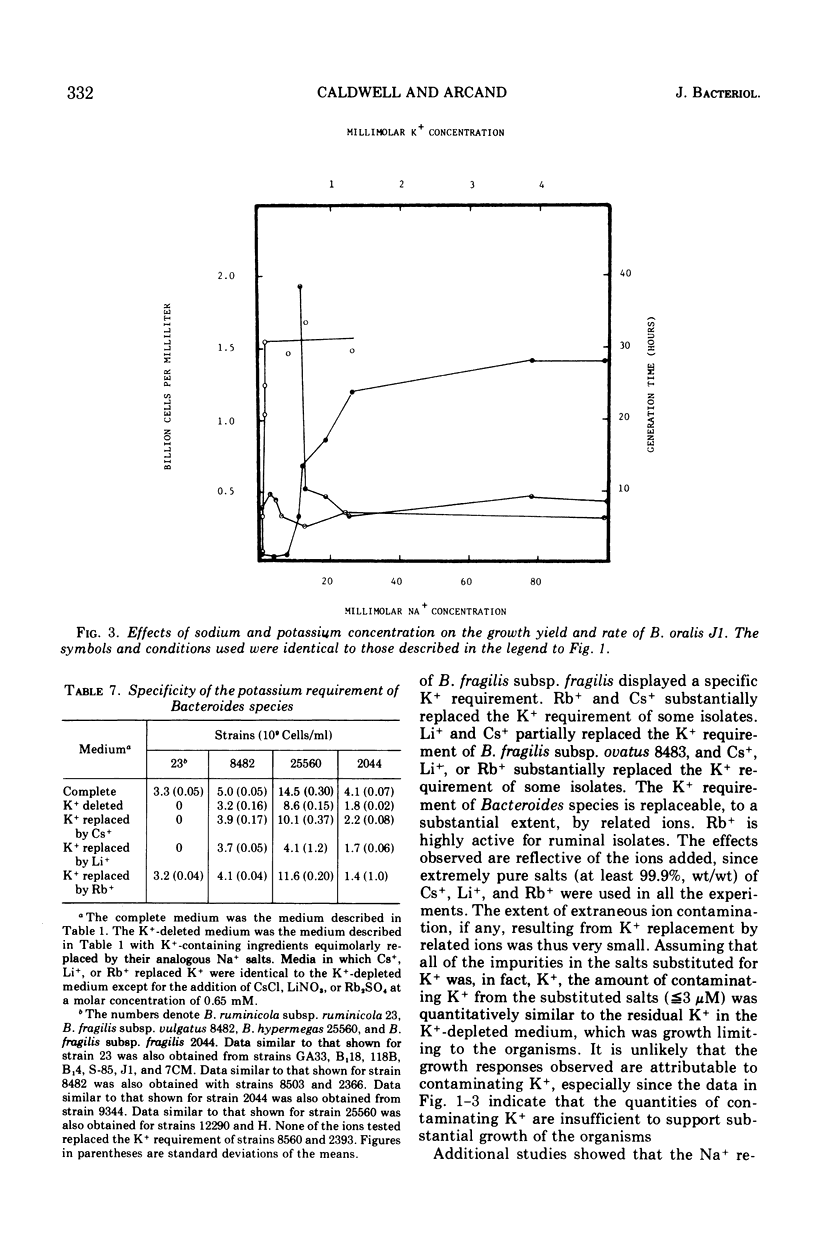
The inorganic and metal-organic growth requirements of ruminal and nonruminal Bacteroides species were compared. The heme requirement of many nonruminal Bacteroides species was similar to that of Bacteroides ruminicola subsp. ruminicola and was a general tetrapyrrole requirement. Some nonruminal Bacteroides species utilized succinate or alpha-ketoglutarate, as well as tetrapyrrole-containing compounds, in place of heme. Fe+ as well as heme was required for maximal yields of some Bacteroides species. The divalent cation requirements of Bacteroides species are complex. Mg2+ deletion from a medium containing Mg2+, Ca2+, Co2+, and Mn2+ reduced the yields of all isolates. Ca2+ deletion from the same medium reduced the growth yields of Bacteroides fragilis, B. fundiliformis, and one strain of B. oralis. The effects of Mg2+ and Ca2+ on the growth of Bacteroides isolates was influenced by other divalent cations. Relatively large quantities of Na+ were obligately required by all of the currently recognized predominant rumen Bacteroides species. Nonruminal Bacteroides species either did not require Na+ or required only small amounts. The Na+ requirement of some nonruminal Bacteroides species could be partially replaced by Li+ or Cs+. The Na+ requirement of rumen Bacteroides species was absolute. The inorganic and metal-organic growth requirements of Bacteroides species appear useful as aids in species differentiation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BRYANT M. P., ROBINSON I. M. Some nutritional characteristics of predominant culturable ruminal bacteria. J Bacteriol. 1962 Oct;84:605–614. doi: 10.1128/jb.84.4.605-614.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., Keeney M., Barton J. S., Kelley J. F. Sodium and other inorganic growth requirements of bacteroides amylophilus. J Bacteriol. 1973 May;114(2):782–789. doi: 10.1128/jb.114.2.782-789.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldwell D. R., White D. C., Bryant M. P., Doetsch R. N. Specificity of the heme requirement for growth of Bacteroides ruminicola. J Bacteriol. 1965 Dec;90(6):1645–1654. doi: 10.1128/jb.90.6.1645-1654.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courant P. R., Gibbons R. J. Biochemical and immunological heterogeneity of Bacteroides melaninogenicus. Arch Oral Biol. 1967 Dec;12(12):1605–1613. doi: 10.1016/0003-9969(67)90194-x. [DOI] [PubMed] [Google Scholar]
- EIKEN M. Studies on an anaerobic, rodshaped, gram-negative microorganism: Bacteroides corrodens n. sp. Acta Pathol Microbiol Scand. 1958;43(4):404–416. [PubMed] [Google Scholar]
- Eggerth A. H., Gagnon B. H. The Bacteroides of Human Feces. J Bacteriol. 1933 Apr;25(4):389–413. doi: 10.1128/jb.25.4.389-413.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GIBBONS R. J., MACDONALD J. B. Hemin and vitamin K compounds as required factors for the cultivation of certain strains of Bacteroides melaninogenicus. J Bacteriol. 1960 Aug;80:164–170. doi: 10.1128/jb.80.2.164-170.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutner S. H. Inorganic nutrition. Annu Rev Microbiol. 1972;26:313–346. doi: 10.1146/annurev.mi.26.100172.001525. [DOI] [PubMed] [Google Scholar]
- Jackson F. L., Goodman Y. E., Bel F. R., Wong P. C., Whitehouse R. L. Taxonomic status of facultative and strictly anaerobic "corroding bacilli" that have been classified as Bacteroides corrodens. J Med Microbiol. 1971 May;4(2):171–184. doi: 10.1099/00222615-4-2-171. [DOI] [PubMed] [Google Scholar]
- LESTER G. Requirement for potassium by bacteria. J Bacteriol. 1958 Apr;75(4):426–428. doi: 10.1128/jb.75.4.426-428.1958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOESCHE W. J., SOCRANSKY S. S., GIBBONS R. J. BACTEROIDES ORALIS, PROPOSED NEW SPECIES ISOLATED FROM THE ORAL CAVITY OF MAN. J Bacteriol. 1964 Nov;88:1329–1337. doi: 10.1128/jb.88.5.1329-1337.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lev M., Keudell K. C., Milford A. F. Succinate as a growth factor for Bacteroides melaninogenicus. J Bacteriol. 1971 Oct;108(1):175–178. doi: 10.1128/jb.108.1.175-178.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesche W. J., Gibbons R. J. A practical scheme for identification of the most numerous oral gram negative anaerobic rods. Arch Oral Biol. 1965 Jul-Aug;10(4):723–725. doi: 10.1016/0003-9969(65)90017-8. [DOI] [PubMed] [Google Scholar]
- Sharpe M. E. Serology of rumen bacteroides. J Gen Microbiol. 1971 Aug;67(3):273–288. doi: 10.1099/00221287-67-3-273. [DOI] [PubMed] [Google Scholar]
- Spiers M. Classification systems of the Bacteroides group. Med Lab Technol. 1971 Oct;28(4):360–366. [PubMed] [Google Scholar]
- Stanley S. O., Morita R. Y. Salinity effect on the maximal growth temperature of some bacteria isolated from marine enviroments. J Bacteriol. 1968 Jan;95(1):169–173. doi: 10.1128/jb.95.1.169-173.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]


