Abstract
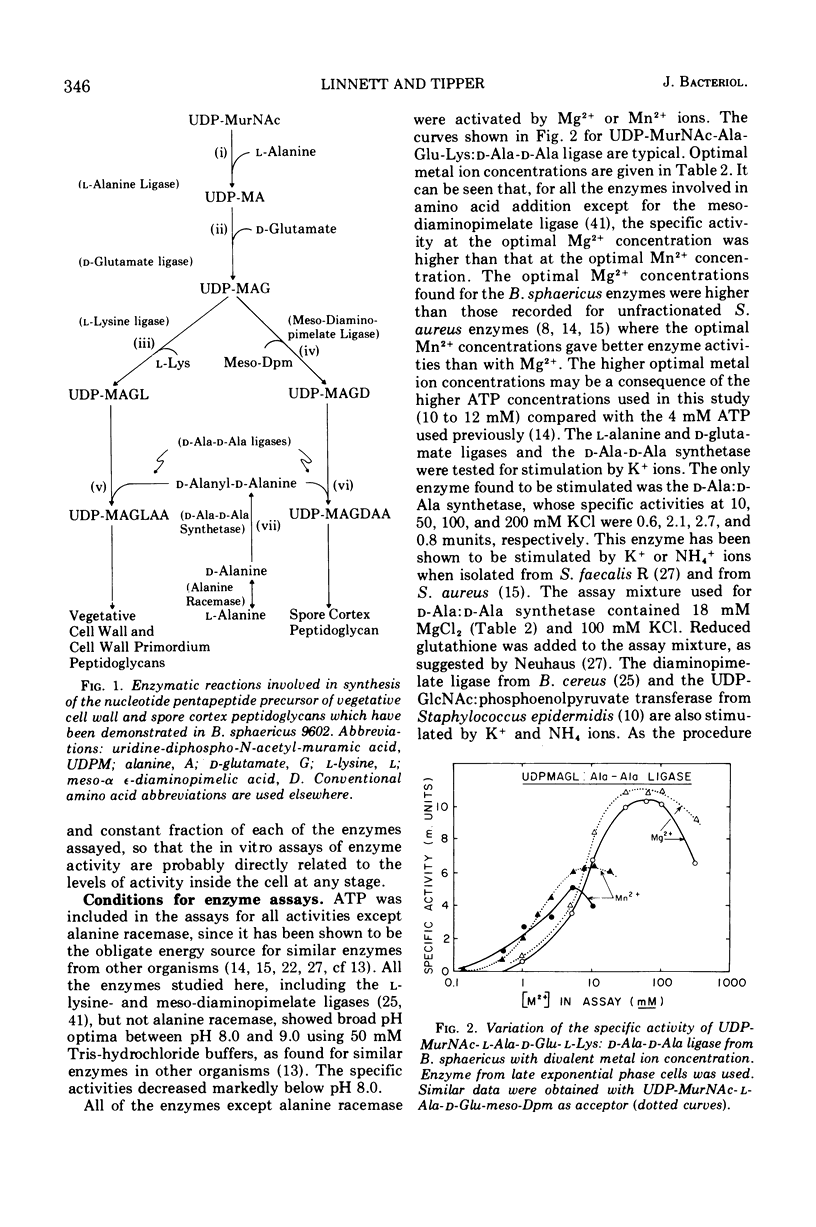
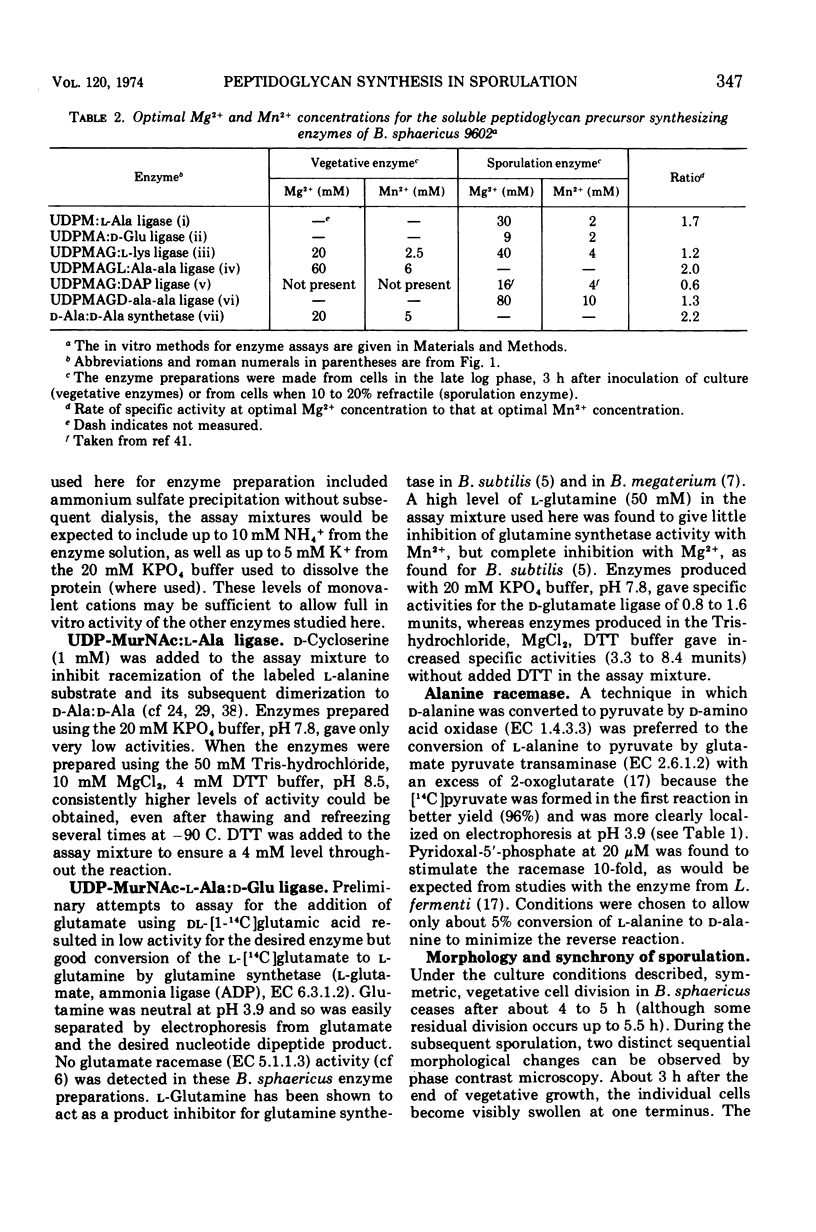
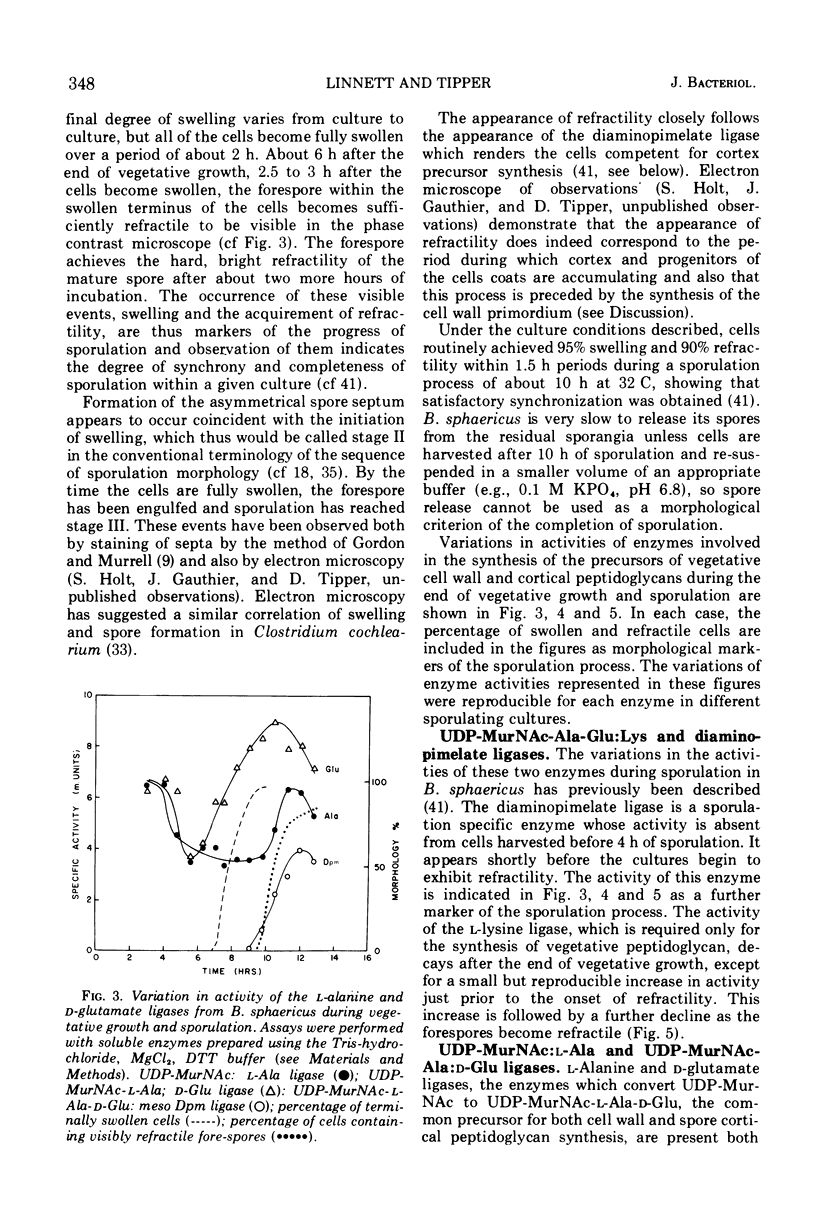
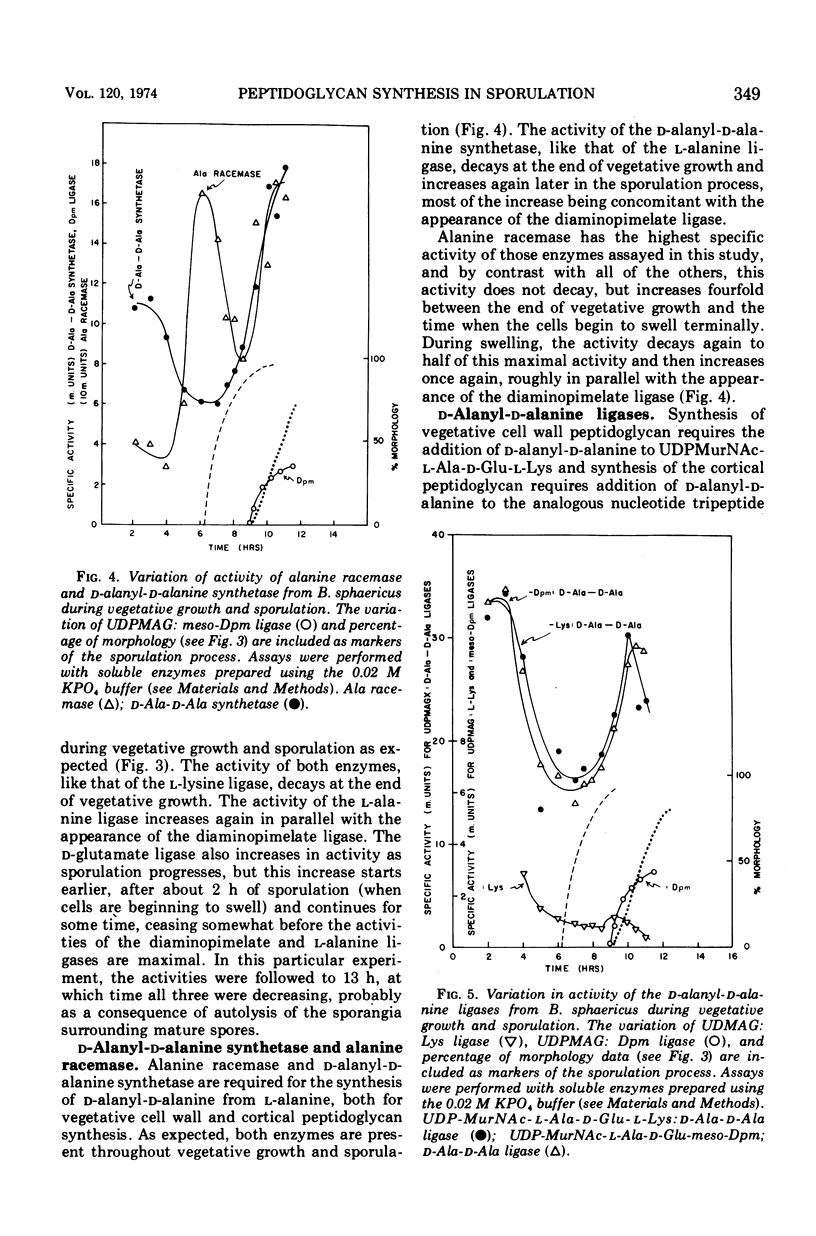
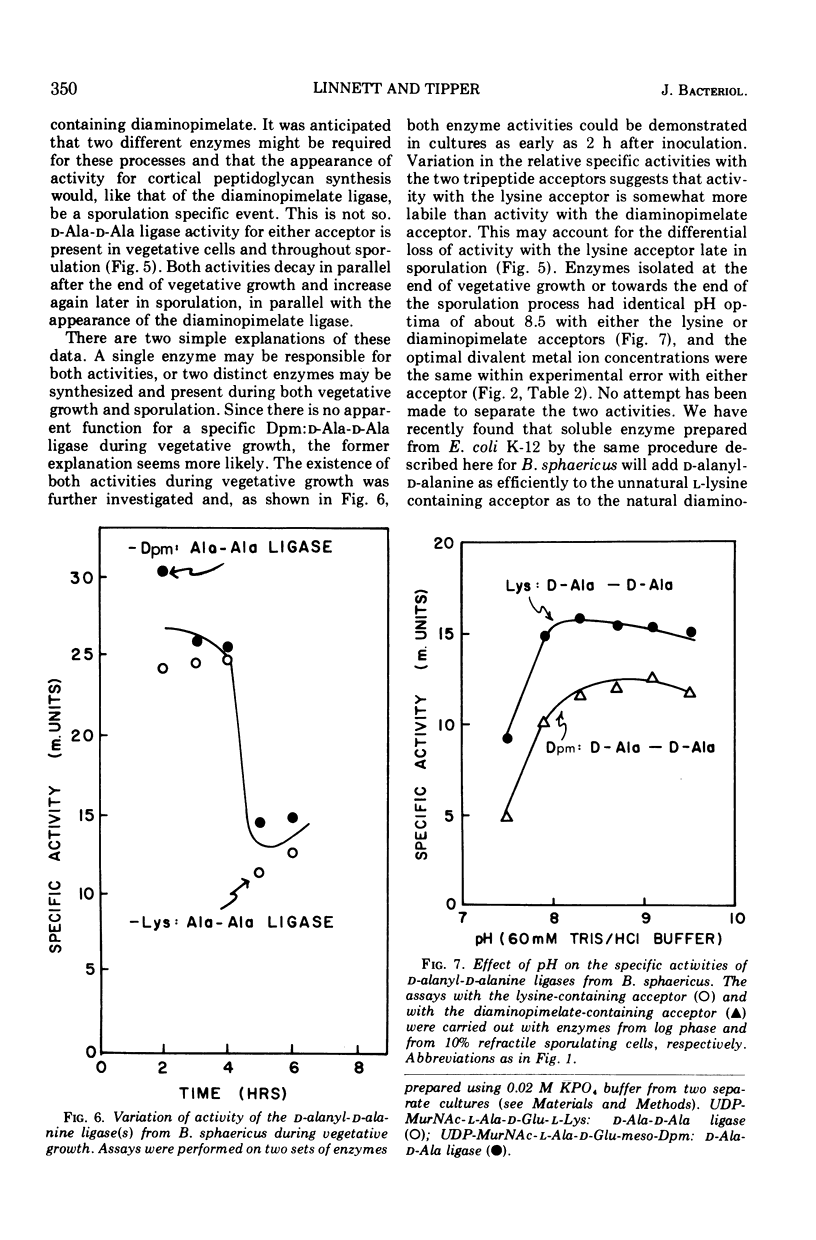
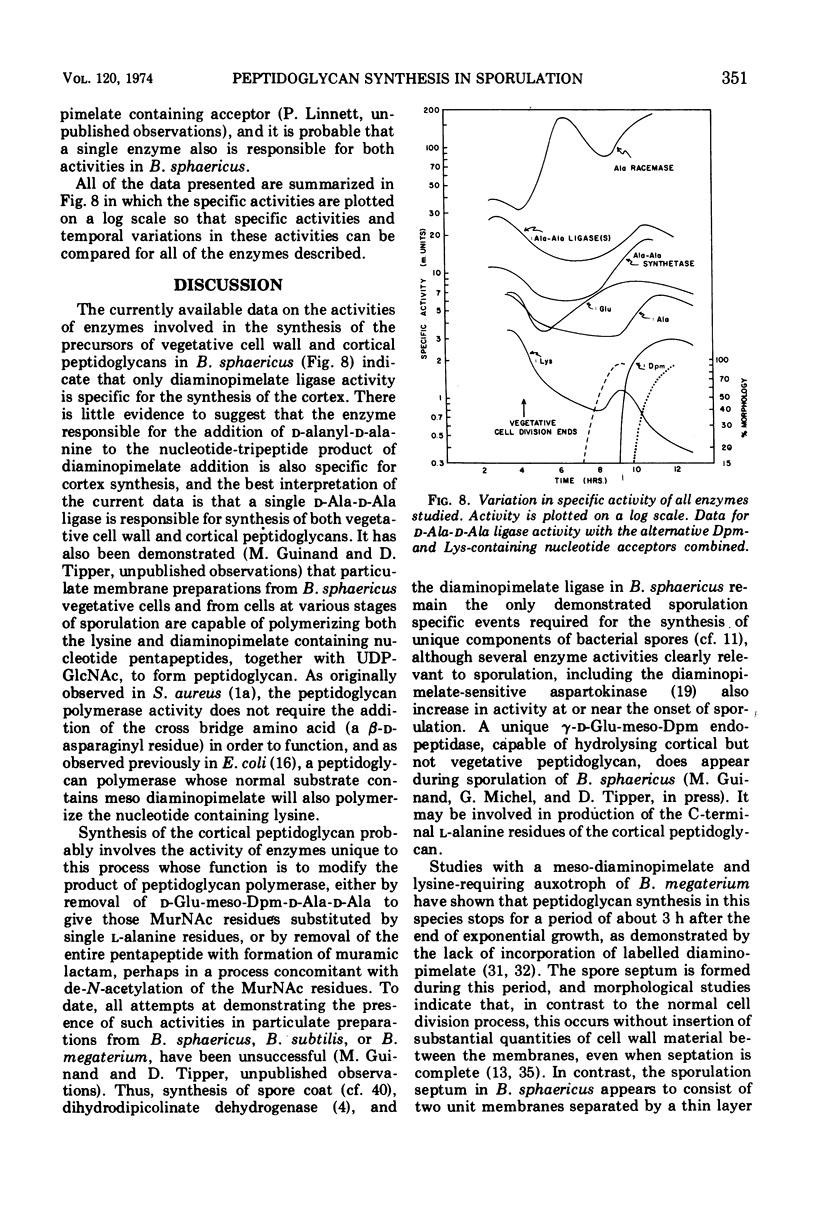
In synchronously sporulating cells of Bacillus sphaericus 9602, the specific activities of those enzymes specifically required for the synthesis of the UDP-N-acetyl-muramyl-pentapeptide precursor of vegetative cell wall peptidoglycan decay by 50% after the end of exponential cell division, probably as a consequence of dilution by newly synthesized protein. The meso-diaminopimelate ligase is the only new activity whose synthesis is required for synthesis of the nucleotide-pentapeptide precursor of spore cortex peptidoglycan. The addition of d-Ala-d-Ala to the nucleotide tripeptide is catalyzed by an enzyme present in both vegetative and sporulating cells, which apparently does not discriminate between lysine- and diaminopimelate-containing acceptors. The activities of the l-Ala and d-Ala-d-Ala ligases and of the d-Ala-d-Ala synthetase increases in parallel with the appearance of the diaminopimelate ligase, indicating coordinate derepression and suggesting operon-like organization of the appropriate structural genes.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson J. S., Meadow P. M., Haskin M. A., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. I. Utilization of uridine diphosphate acetylmuramyl pentapeptide and uridine diphosphate acetylglucosamine for peptidoglycan synthesis by particulate enzymes from Staphylococcus aureus and Micrococcus lysodeikticus. Arch Biochem Biophys. 1966 Sep 26;116(1):487–515. doi: 10.1016/0003-9861(66)90056-7. [DOI] [PubMed] [Google Scholar]
- Aubert J. P., Millet J. Etude d'une L-leucyl-beta-naphtylamide hydrolase en relation avec la sporulation chez Bacillus megaterium. C R Acad Sci Hebd Seances Acad Sci D. 1965 Nov 15;261(20):4274–4277. [PubMed] [Google Scholar]
- Deuel T. F., Stadtman E. R. Some kinetic properties of Bacillus subtilis glutamine synthetase. J Biol Chem. 1970 Oct 25;245(20):5206–5213. [PubMed] [Google Scholar]
- Diven W. F. Studies on amino acid racemases. II. Purification and properties of the glutamate racemase from Lactobacillus fermenti. Biochim Biophys Acta. 1969;191(3):702–706. doi: 10.1016/0005-2744(69)90364-7. [DOI] [PubMed] [Google Scholar]
- Elmerich C. Le cycle du glutamate, point de départ du métabolisme de l'azote, chez Bacillus megaterium. Eur J Biochem. 1972 May 23;27(2):216–224. doi: 10.1111/j.1432-1033.1972.tb01829.x. [DOI] [PubMed] [Google Scholar]
- Good C. M., Tipper D. J. Conditional mutants of Staphylococcus aureus defective in cell wall precursor synthesis. J Bacteriol. 1972 Jul;111(1):231–241. doi: 10.1128/jb.111.1.231-241.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. A., Murrell W. G. Simple method of detecting spore septum formation and synchrony of sporulation. J Bacteriol. 1967 Jan;93(1):495–496. doi: 10.1128/jb.93.1.495-496.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunetileke K. G., Anwar R. A. Biosynthesis of uridine diphospho-N-acetyl muramic acid. J Biol Chem. 1966 Dec 10;241(23):5740–5743. [PubMed] [Google Scholar]
- Hammes W. P., Neuhaus F. C. On the specificity of phospho-N-acetylmuramyl-pentapeptide translocase. The peptide subunit of uridine diphosphate-N-actylmuramyl-pentapeptide. J Biol Chem. 1974 May 25;249(10):3140–3150. [PubMed] [Google Scholar]
- Hanson R. S., Peterson J. A., Yousten A. A. Unique biochemical events in bacterial sporulation. Annu Rev Microbiol. 1970;24:53–90. doi: 10.1146/annurev.mi.24.100170.000413. [DOI] [PubMed] [Google Scholar]
- Hungerer K. D., Tipper D. J. Cell wall polymers of Bacillus sphaericus 9602. I. Structure of the vegetative cell wall peptidoglycan. Biochemistry. 1969 Sep;8(9):3577–3587. doi: 10.1021/bi00837a013. [DOI] [PubMed] [Google Scholar]
- Izaki K., Matsuhashi M., Strominger J. L. Biosynthesis of the peptidoglycan of bacterial cell walls. 8. Peptidoglycan transpeptidase and D-alanine carboxypeptidase: penicillin-sensitive enzymatic reaction in strains of Escherichia coli. J Biol Chem. 1968 Jun 10;243(11):3180–3192. [PubMed] [Google Scholar]
- Johnston M. M., Diven W. F. Studies on amino acid racemases. I. Partial purification and properties of the alanine racemase from Lactobacillus fermenti. J Biol Chem. 1969 Oct 10;244(19):5414–5420. [PubMed] [Google Scholar]
- Kay D., Warren S. C. Sporulation in Bacillus subtilis. Morphological changes. Biochem J. 1968 Oct;109(5):819–824. doi: 10.1042/bj1090819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuramitsu H. K., Yoshimura S. Elevated diaminopimelate-sensitive aspartokinase activity during sporulation of Bacillus stearothermophilus. Biochim Biophys Acta. 1972 Mar 30;264(1):152–164. doi: 10.1016/0304-4165(72)90126-2. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lugtenberg E. J., De Haas-Menger L., Ruyters W. H. Murein synthesis and identification of cell wall precursors of temperature-sensitive lysis mutants of Escherichia coli. J Bacteriol. 1972 Jan;109(1):326–335. doi: 10.1128/jb.109.1.326-335.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lugtenberg E. J. Studies on Escherichia coli enzymes involved in the synthesis of uridine diphosphate-N-acetyl-muramyl-pentapeptide. J Bacteriol. 1972 Apr;110(1):26–34. doi: 10.1128/jb.110.1.26-34.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch J. L., Neuhaus F. C. On the mechanism of action of the antibiotic O-carbamyld-serine in Streptococcus faecalis. J Bacteriol. 1966 Jan;91(1):449–460. doi: 10.1128/jb.91.1.449-460.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizuno Y., Ito E. Purification and properties of uridine diphosphate N-acetylmuramyl-L-alanyl-D-glutamate:meso-2,6-diaminopimelate ligase. J Biol Chem. 1968 May 25;243(10):2665–2672. [PubMed] [Google Scholar]
- NEUHAUS F. C., LYNCH J. L. THE ENZYMATIC SYNTHESIS OF D-ALANYL-D-ALANINE. 3. ON THE INHIBITION OF D-ALANYL-D-ALANINE SYNTHETASE BY THE ANTIBIOTIC D-CYCLOSERINE. Biochemistry. 1964 Apr;3:471–480. doi: 10.1021/bi00892a001. [DOI] [PubMed] [Google Scholar]
- NEUHAUS F. C. The enzymatic synthesis of D-alanyl-D-alanine. I. Purification and properties of D-alanyl-D-alanine synthetase. J Biol Chem. 1962 Mar;237:778–786. [PubMed] [Google Scholar]
- Neuhaus F. C., Carpenter C. V., Miller J. L., Lee N. M., Gragg M., Stickgold R. A. Enzymatic synthesis of D-alanyl-D-alanine. Control of D-alanine:D-alanine ligase (ADP). Biochemistry. 1969 Dec;8(12):5119–5124. doi: 10.1021/bi00840a066. [DOI] [PubMed] [Google Scholar]
- Osborn M. J. Structure and biosynthesis of the bacterial cell wall. Annu Rev Biochem. 1969;38:501–538. doi: 10.1146/annurev.bi.38.070169.002441. [DOI] [PubMed] [Google Scholar]
- Pitel D. W., Gilvarg C. Mucopeptide metabolism during growth and sporulation in Bacillus megaterium. J Biol Chem. 1970 Dec 25;245(24):6711–6717. [PubMed] [Google Scholar]
- Pitel D. W., Gilvarg C. Timing of mucopeptide and phospholipid synthesis in sporulating Bacillus megaterium. J Biol Chem. 1971 Jun 10;246(11):3720–3724. [PubMed] [Google Scholar]
- Pope L., Rode L. J. Spore fine structure in Clostridium cochlearium. J Bacteriol. 1969 Nov;100(2):994–1001. doi: 10.1128/jb.100.2.994-1001.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prestidge L., Gage V., Spizizen J. Protease activities during the course of sporulation on Bacillus subtilis. J Bacteriol. 1971 Sep;107(3):815–823. doi: 10.1128/jb.107.3.815-823.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RYTER A. ETUDE MORPHOLOGIQUE DE LA SPORULATION DE BACILLUS SUBTILIS. Ann Inst Pasteur (Paris) 1965 Jan;108:40–60. [PubMed] [Google Scholar]
- Schleifer K. H., Kandler O. Peptidoglycan types of bacterial cell walls and their taxonomic implications. Bacteriol Rev. 1972 Dec;36(4):407–477. doi: 10.1128/br.36.4.407-477.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tipper D. J., Pratt I. Cell wall polymers of Bacillus sphaericus 9602. II. Synthesis of the first enzyme unique to cortex synthesis during sporulation. J Bacteriol. 1970 Aug;103(2):305–317. doi: 10.1128/jb.103.2.305-317.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan from spores of Bacillus subtilis. Biochemistry. 1972 Apr 11;11(8):1389–1396. doi: 10.1021/bi00758a010. [DOI] [PubMed] [Google Scholar]
- Warth A. D., Strominger J. L. Structure of the peptidoglycan of bacterial spores: occurrence of the lactam of muramic acid. Proc Natl Acad Sci U S A. 1969 Oct;64(2):528–535. doi: 10.1073/pnas.64.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]


