Abstract
Central nervous system (CNS) damage and dysfunction are devastating consequences of HIV infection. Although the CNS is one of the initial targets for HIV infection, little is known about early viral-induced abnormalities that can affect CNS function. Here we report the detection of early physiological abnormalities in simian immunodeficiency virus-infected monkeys. The acute infection caused a disruption of the circadian rhythm manifested by rises in body temperature, observed in all five individuals between 1 and 2 weeks postinoculation (p.i.), accompanied by a reduction in daily motor activity to 50% of control levels. Animals remained hyperthermic at 1 and 2 months p.i. and returned to preinoculation temperatures at 3 months after viral inoculation. Although motor activity recovered to baseline values at 1 month p.i., activity levels then decreased to approximately 50% of preinoculation values over the next 2 months. Analysis of sensory-evoked responses 1 month p.i. revealed distinct infection-induced changes in auditory-evoked potential peak latencies that persisted at 3 months after viral inoculation. These early physiological abnormalities may precede the development of observable cognitive or motor deficiencies and can provide an assay to evaluate agents to prevent or alleviate neuronal dysfunction.
Recent therapeutic advances have dramatically reduced blood-borne and lymphoid organ HIV load (1–3). However, HIV infection of the central nervous system (CNS), which has been documented to occur within the first month of HIV and simian immunodeficiency virus (SIV) infection (4–7), presents unique therapeutic problems. Cells of the macrophage lineage are infected in the CNS, and macrophages can be a major source of productive virus in the later stages of disease (8). Because most of the anti-HIV agents do not cross the blood–brain barrier, the CNS may remain a reservoir for HIV as well as be uniquely susceptible to continued damage over the prolonged course of HIV infection resulting from systemic therapies.
The progressive motor and cognitive abnormalities found in HIV-infected individuals are believed to be initiated by infection of the CNS by HIV itself (9). Yet the cascade of events in the CNS leading to neuropsychological impairment, motor disorders, and even frank dementia remains unknown. One step toward unveiling such mechanisms is to analyze the temporal relationship between viral infection and the time course of specific physiological changes to identify critical periods of the disease progression. In HIV-infected individuals, multiple physiological parameters such as sleep patterns, psychomotor performance, and visual-, auditory-, and somatosensory-evoked potentials are altered (10–16). Such physiologic dysregulations can have important consequences for cognitive functions (17, 18). Knowledge of the time of onset of such physiological changes could lead to the establishment of event markers in SIV disease progression that may be useful criteria for evaluating pharmacotherapies.
We hypothesize that after immunodeficiency virus infection, CNS function can be altered by viral-induced cellular alterations and the host’s response to infection, resulting in early measurable changes of physiological parameters such as body temperature, gross motor activity, sleep, and evoked potentials. SIV has been shown to cause an AIDS-like immune deficiency and CNS infection when inoculated into rhesus monkeys (19, 20). We and others have demonstrated functional CNS deficiencies in SIV-infected rhesus monkeys, including abnormalities in sensory-evoked potentials, behavioral/cognitive abnormalities, and motor problems (21–23). Here we focus on the examination of multiple physiological parameters during the early states of SIV infection of rhesus monkeys. Within the first 3 months after viral inoculation, distinct changes in body temperature, motor activity, and evoked potentials have been identified. These alterations can serve as sensitive indicators for physiological abnormalities induced in monkeys by SIV, as a model for human infection by HIV.
METHODS
Subjects.
The five male rhesus monkeys (Charles River Breeding Laboratories) used were free of type D simian retroviruses and herpes B virus. The monkeys were individually housed in stainless-steel cages (32 in × 28 in × 31 in), which permit olfactory, visual, and auditory contact with other monkeys in the room while minimizing tactile contact. Room conditions include a light/dark cycle of 6 a.m. to 6 p.m. light and a room temperature of 24.5 ± 2.5°C. Blood and cerebrospinal fluid (CSF) sampling were performed preinoculation, and at 1, 2, 4, 6, 8, and 12 weeks postinoculation (p.i.) in ketamine-immobilized animals by using the femoral vein and cisterna magna, respectively.
All animal work was approved by The Scripps Research Institute’s Animal Care Committee. The Scripps Research Institute is accredited by the American Association for the Accreditation of Laboratory Animal Care.
Viral Inoculation.
Viral inoculation was performed after a stable baseline of circadian body temperature and motor activity were established. For SIV infection, animals were inoculated by saphenous vein injection of 0.5 ml of a cryopreserved stock of cell-free SIV [p27 (gag) antigen equivalent = 1.1 ng/ml] derived from serial passage of the SIVmac251 strain through microglia (24–26). All animals became productively infected as evidenced by viral recovery from the animals’ peripheral blood mononuclear cells. For comparison of results from the studies, data collected before viral inoculation are labeled preinoculation, 4–5 weeks p.i. are referred to as 1 month, 8 weeks p.i. as 2 months, and 12 weeks p.i. as 3 months p.i. Necropsies were performed as described (22). Monkey no. 260 was sacrificed at 17 weeks p.i. because of inactivity and weight loss, with autopsy revealing SIV encephalitis and Pneumocystis carinii pneumonia. Monkey no. 266 was sacrificed at 28 weeks p.i. because of a depressive affect and tremulous movements, with autopsy revealing SIV encephalitis and SIV pneumonitis. Monkey no. 271 was sacrificed at 47 weeks p.i. because of weakness, ataxia, anorexia, and a depressive affect, with autopsy revealing lymphoma involving the trigeminal ganglia, dorsal root ganglia, subarachnoid space, focal infiltration of the brain parenchyma, and spleen and lymph nodes. Foci of activated macrophages were also present in the CNS. Monkey no. 264 was sacrificed per experimental protocol at 11 weeks p.i. and showed lymphoid hyperplasia in addition to foci of perivascular inflammation in the CNS at autopsy. Monkey no. 270 was sacrificed per experimental protocol at 84 weeks p.i. and showed lymphoid hyperplasia in addition to foci of perivascularly located, activated macrophages in the CNS at autopsy.
Telemetry.
Telemetry transmitters (TA 10 CTA-D70, capable of detecting body temperature, gross motor activity, and one biopotential), antenna plates (Receiverplate RLA 2000), and the hardware and software of the data acquisition system were purchased from Datasciences, St. Paul, MN. Aseptic surgical implantation of the telemetry transmitters was performed in anesthetized animals through a transverse incision in the animals’ flank 2 cm caudal of the right scapula, and a s.c. pocket was bluntly dissected in caudal direction to house the implant. The biopotential leads were attached to cranial screws. To place the screws, a 3-cm incision was made at the vertex, and the cranium was exposed. Two stainless-steel screws were placed through the cranium extending to the dura. The first was inserted 1 cm lateral to the vertex and the second 2 cm lateral to the first. Leads from the transmitter were tunneled s.c., wrapped around the screws, and secured in place with dental cement. After surgical implantation, the animals were allowed at least 3 weeks recovery before the start of data acquisition.
Motor Activity and Body Temperature.
Cages were equipped with individual antennas, which in turn were connected to the data acquisition computer located in a separate room. The telemetry data acquisition system was programmed to take readings from each monkey at 10-min intervals, a motor activity count per 10-min interval, a temperature reading (10-sec average at 200-Hz sampling frequency), and a 30-sec electroencephalogram (EEG) epoch (40-Hz low pass filter, 120-Hz sampling frequency). Data were analyzed by using the analysis feature of the data acquisition program dataquest followed by statistical analysis using Microsoft excel and Dynamic Microsystems’ gb-stat. Statistical comparisons were made by using preinoculation data of each animal as controls, and significance was tested by using an ANOVA followed by post hoc t-paired tests when the ANOVA indicated significance.
EEG.
Daylight and IR video monitoring of the animals’ behavior in conjunction with on-line EEG recording was performed before the experiment to calibrate the recording system to enable the identification of slow-wave sleep. In brief, analog output interface was connected to the telemetry setup, and the analog EEG signal was fed into an Grass 12A5 ac amplifier, filtered with a bandwidth 0.1–100 Hz, and visualized on a polygraph. EEG and behavior were videotaped simultaneously. Identified artifacts were cataloged, and EEG epochs displaying such patterns were discarded from analysis. For each animal, EEG power spectrum analysis (0.25-Hz intervals) was performed on the EEG data of an 8-hr interval starting 2 hr after onset of the dark cycle. Analysis was performed on recordings before inoculation, 4 weeks (referred to as 1 month) p.i. and at the day of the highest fever in the first 2 weeks p.i. The power spectrum for each acquired epoch during the 8-hr period was computed, and an average power spectrum for the whole period was calculated.
Evoked Potentials.
Cortical auditory evoked potentials (AEPs) and brainstem AEPs (BSAEPs) were evaluated in all five subjects as described (22). In brief, ketamine (20 mg/kg) immobilized monkeys were aseptically fitted with acute s.c. monopolar needle electrodes. The two active electrodes were placed centrally near the vertex and on the midline 1 cm above the nasion, respectively. One additional electrode (ground) was placed in the nuchal muscles. Computer programs were used to generate stimuli. Evoked events were analyzed off-line by using computer-based signal averaging. The data from each session were graphed and waves were identified by visual inspection. Averaged peak latencies were calculated and compared between groups. Statistical differences were determined by using an ANOVA followed by Student’s paired t test.
Integrity of the Blood–Brain Barrier.
Albumin concentrations in both plasma and CSF were quantified in the animals by using a radial immunodiffusion test kit (The Binding Site, San Diego). The red blood cell (RBC) count of the CSF was determined as a measure of blood contamination, and samples exceeding 10,000 RBC/μl were discarded from analysis (samples from four of the animals were assessed; repeated RBC contamination precluded analysis of samples from the fifth monkey). The albumin quotient (CSF albumin concentration/serum albumin concentration) was calculated as a measure for blood–brain barrier integrity.
RESULTS
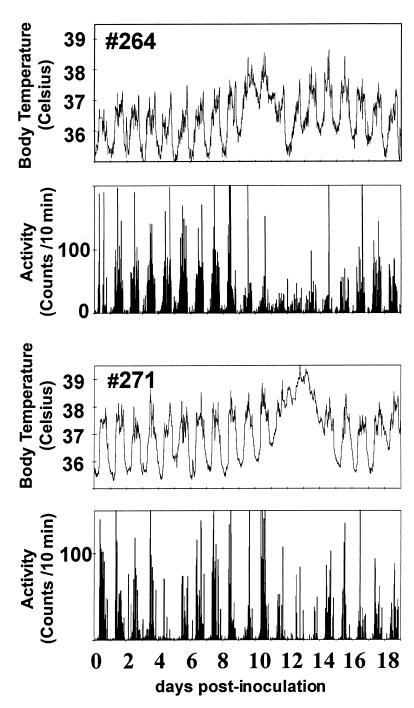
To obtain comprehensive information on the effect of infection on the animals’ temperature and movement, we adapted telemetric monitoring (27) to the SIV-rhesus monkey model of AIDS. Continuous temperature and motor activity recordings of five monkeys for the 2 weeks before inoculation revealed a consistent circadian rhythm with a daily average temperature of 36.5 ± 0.4°C, a maximum of 37.7 ± 0.3°C during the day and a minimum of 35.3 ± 0.5°C at night. Motor activity also showed distinct day/night patterns with little or no activity during the night. After viral inoculation, changes in both body temperature and motor activity were found. Individual body temperature traces increased between 8 and 13 days p.i. (e.g., Fig. 1). After the initial rise, average body temperature stayed elevated for 3–4 days, peaking at 38.9° ± 0.5°C, which resulted in a maximum daily average temperature of 37.4 ± 0.3°C on day 13 p.i. (P < 0.05 relative to preinoculation average). Concomitant with the increase in temperature, the circadian rhythm of the temperature was altered in that the differences between maximum and minimum body temperature were decreased during the occurrence of the fever in all animals.
Figure 1.
Radiotelemetric measurement of movement and temperature after SIV inoculation of two rhesus monkeys (nos. 264 and 271). Temperature traces and gross motor activity traces of monkey no. 264 (Upper) and monkey no. 271 (Lower).
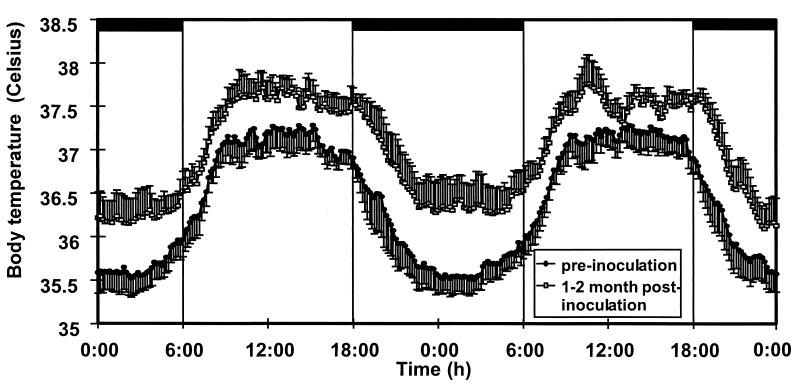
Daily average body temperature decreased after the fever peak. However, average body temperature did not completely return to baseline. Analysis of the circadian temperature rhythm before inoculation and over the period of 1–2 months p.i. revealed a constant hyperthermic shift throughout the day (Fig. 2) to 0.6° above normalized preinoculation baseline values. The temperature remained elevated at 2 months p.i. before returning to near baseline values at 3 months after viral inoculation.
Figure 2.
Circadian rhythm of body temperature in rhesus monkeys before and after infection with SIV. Averages of temperature traces of five rhesus monkeys. For each animal an average circadian rhythm curve from data obtained over two 48-hr periods was calculated for the period before inoculation and for the period between 1 and 2 months p.i. The depicted curves represent the grand average of five monkeys. Data are shown with the SEM. Bars indicate the time of room darkness.
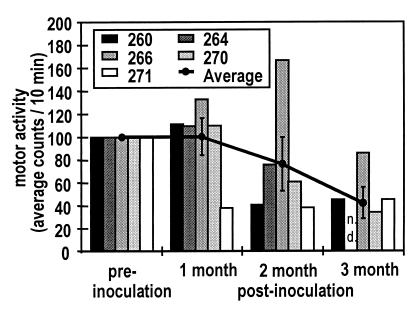
The individual gross motor activity counts decreased in all five animals after inoculation during and/or immediately after the period of fever (e.g., Fig. 1). In the five monkeys, the average daily activity levels decreased, dropping to a level of approximately 50% of preinoculation levels on day 11 p.i. (P < 0.01). This decrease also was associated with a loss of the circadian motor activity rhythm with less distinct day versus night contrast. In contrast to the body temperature data, longitudinal analysis revealed a recovery of the normalized average motor activity at 1 month p.i. However, the average motor activity began to show a decrease at 2 months p.i. and was significantly decreased (by 47%) at 3 months p.i. (Fig. 3).
Figure 3.
Individual (bars) and averaged (line) normalized gross motor activity measurements of five monkeys before, 1, 2, and 3 months after inoculation with SIV. At 3 months p.i. the decrease in motor activity reaches statistical significance when compared with preinoculation levels (ANOVA with post hoc t test, P < 0.05. Comparisons are made within subjects, n = 5 for preinoculation, 1 and 2 months. Monkey no. 264 was sacrificed at 11 weeks after SIV inoculation, therefore n = 4 at 3 months).
Because EEG slow-wave power during sleep is abnormal in HIV-infected individuals (10) EEG slow-wave power was analyzed in the SIV-infected monkeys. To assess whether early sleep abnormalities also could be detected, observational analysis was combined with EEG recordings during the 2 hr after the initiation of the dark cycle. Examination of the EEG during periods of no movement revealed a clear slow-wave pattern in the EEG, indicating slow-wave sleep. Power spectrum analysis showed highly similar power in slow-wave sleep recordings across days before inoculation. Although the 1-Hz slow-wave power decreased with a minimum at the time of the highest fever after the inoculation (before inoculation, 0.27 ± 0.031 mV2/Hz; point of highest fever, 0.22 ± 0.029 mV2/Hz, P < 0.05), no significant changes were found at low frequency intervals later in the course of infection (data not shown).
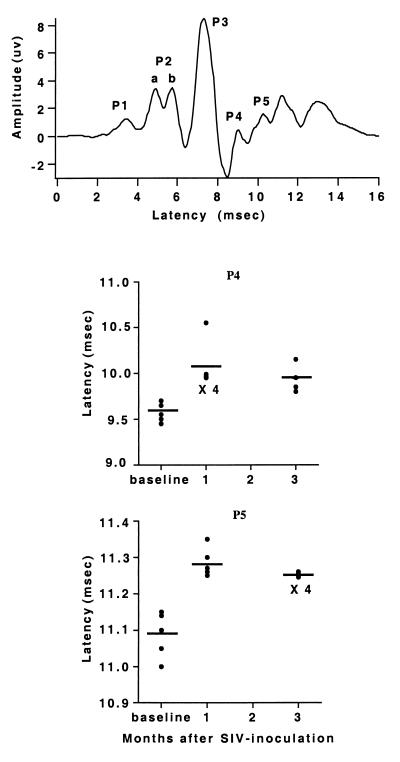
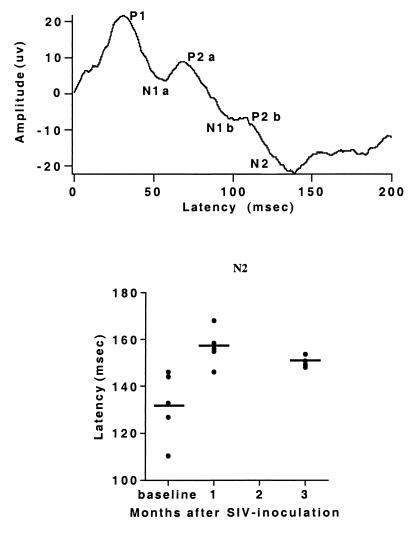
Sensory-evoked potentials were obtained preinoculation, and at 1 and 3 months p.i. Five waves, P1-P5, were identified in the BSAEP complex (the P2 wave had two peaks, labeled P2a and P2b as shown). The BSAEP peak latencies of P1-P3 from SIV-infected animals did not differ from the preinoculation recordings, but showed significant delays of waves P4 and P5 at 1 month p.i., which persisted at 3 months p.i. (Fig. 4). The long-latency cortical AEPs of infected monkeys displayed three positive (P1, P2a, and P2b) and three negative peaks (N1a, N1b, and N2). N2 occurred with a significant delay at 1 month p.i. as compared with preinoculation recordings, and this delay also persisted at 3 months p.i. (Fig. 5). Additionally, at 3 months p.i., the latency of P2b was significantly delayed (data not shown). The early and consistent delays in P4 and P5 in the BSAEPs and in N2 in the AEPs, were similar to those previously found in chronically SIV-infected monkeys (22).
Figure 4.
BSAEP latencies. (Upper) Representative waveform tracing. (Lower) Plots of individual values (circles) and averages (lines) of the latency of P4 and P5. The P4 and P5 latencies measured at both 1 and 3 months after inoculation are significantly different than preinfection values (ANOVA with post hoc t test, P < 0.05).
Figure 5.
AEP latencies. (Upper) Representative waveform tracing. (Lower) Plot of individual values (circles) and averages (lines) of the latency of N2. The N2 latency measured at both 1 and 3 months after inoculation is significantly different than the preinfection value (ANOVA with post hoc t test, P < 0.01).
In rhesus monkeys and humans, SIV- or HIV-infected cells have been found in the CNS early after i.v. inoculation (4–7). Although an analysis of viral infection of the CNS parenchyma in these monkeys would preclude the performance of physiological studies, in situ hybridization analysis was performed in the brains of two acutely infected macaques. The first animal (no. 272) was sacrificed before any increase in body temperature was noted by telemetry (7 days p.i.), and the second on the day of the initial rise of body temperature after inoculation (12 days p.i. for this animal). Cells positive for SIV RNA could be found in the CNS of the latter animal only, thus coinciding with the initial rise in body temperature (T.F.W.H. and H.S.F., unpublished observations). Studies examining viral parameters in rhesus monkeys have revealed that the peak of systemic viral load after i.v. inoculation occurs between 9 and 13 days p.i. (28). Consistent with these data, plasma viral load at sacrifice (measured by the Chiron b-DNA assay) in animal no. 267 at 12 days p.i. was more than 400 times greater than that in animal no. 272 at 7 days p.i. (H.S.F., unpublished observations).
Because breakdown of the blood–brain barrier breakdown has been reported during infectious CNS diseases, including human (29, 30) and simian (31) AIDS, we examined whether blood–brain barrier leakage, measured by the CSF albumin quotient, could be detected in these animals. The CSF albumin quotient of two of four animals examined did rise above the range of control values (normal albumin quotient <0.005, monkey no. 260 at 1 week and monkey no. 270 at 6 weeks p.i. both had albumin quotients = 0.02), indicating that at least a transient leakage of the blood–brain barrier can occur after SIV inoculation.
DISCUSSION
The present data provide a clear indication that SIV infection in rhesus monkeys causes physiological abnormalities that can be detected within the first month after virus inoculation and continue during the subsequent progression of the infection. The bulk of the evidence obtained to date suggests that the initial and progressively declining neurological and cognitive status reported in HIV-1-infected individuals develops as a consequence of the indirect actions of virus-infected non-neuronal cells on neuronal tissue. How the proposed direct or indirect actions of the virus might cause HIV-associated dementia remains the subject of widespread conjecture. The most likely primary cellular targets of the virus are CNS microglia/macrophages, either resident in the brain or having migrated into the CNS from the blood. A variety of inducible vectors that may result in perturbed CNS function have been suggested, including viral-induced release of cytokines from macrophages and/or microglia. Furthermore, a possible “functional” neuronal toxicity induced by either locally released viral protein products or host defense molecules, working through mechanisms including induction of oxidative stress and excitotoxicity, also has been suggested (32, 33).
After HIV infection, many individuals develop an acute, mononucleosis-like illness with fever, lymphadenopathy, malaise, and/or headache. Moreover it has been demonstrated that fever in combination with arthralgias is highly predictive of a recent HIV infection (34). We were able to observe corresponding temperature rises and decreases in motor activity within the first 2 weeks after acute SIV inoculation, which corresponded to the period in which systemic viral load peaks and virus enters the brain. Animals remained hyperthermic at 1 and 2 months p.i. and returned to preinoculation temperatures at 3 months p.i., demonstrating that the thermal regulation of SIV-inoculated animals is altered for a period longer than the initial acute viremia. However, it must be noted that the temperature-sensitive implants were placed s.c., and it is possible that alterations in heat exchange, e.g., because of blood flow changes, may contribute to the temperature differences. Because both fevers and hypothermia identified by these s.c. implanted telemetry devices correspond to alterations in rectally determined body temperature, it is likely that the temperature profiles measured indeed reflect alterations in body temperature. Furthermore, it is unlikely that the increase in daily average temperatures is a result of thermal energy generated by higher muscle activity, because motor activity was unchanged or reduced at these time points. Such temperature alterations might be an indicator of altered biochemical profiles, such as cytokine production, and can contribute to changes of other physiological parameters (35–37). Temperature alterations may contribute to the sleep and fatigue problems reported by HIV-infected individuals and to the development of cognitive and motor abnormalities. A variety of bioelectrical signals are sensitive to temperature, such as axonal conduction, transmitter release, and action potential parameters (38). Because such parameters can affect learning, memory, and autonomic regulation, the temperature changes after SIV inoculation observed here may affect general brain functions.
Although motor activity recovered to baseline values at 1 month p.i., activity levels then decreased to approximately 50% of preinoculation values by 3 months after inoculation. CNS disease in HIV infection frequently is associated with psychomotor slowing as well as impaired fine and gross motor function (12, 13, 16). In a study of cognitive and motor impairment in SIV-infected rhesus monkeys, motor skills were the most consistent impairment found (21, 39). We also have found that SIV infection can result in transient periods of poor performance over the course of infection as well as sustained abnormalities in the later stages of disease (23).
Recordings of evoked potentials are considered to be simple and informative methods to test general CNS function. In contrast to EEG recordings, which measure spontaneous activity, evoked potentials are responses to a defined external stimulus resulting in a local change in the electrical field of a neural structure. We have found that the electrophysiological data collected under these conditions (including ketamine immobilization and monthly cisterna magna CSF taps) are extremely stable over time. For example, the BSAEP P5 latency averaged (±SEM) 10.998 ± 0.0346 and 10.979 ± 0.0172 milliseconds in two animals examined monthly over 13 and 14 months, respectively (S.H.-R. and S.J.H., unpublished data). As we reported earlier (22), chronically infected (37–52 weeks p.i.) macaques show changes in sensory-evoked potentials when compared with uninfected controls. Here we expand these findings and report that changes in the auditory-evoked potentials can be observed as early as 1 month after viral inoculation. Significantly, despite different disease progressions in the individual animals, we find that SIV inoculation induces similar alterations in the animals’ evoked potentials.
Delays in these late components of the BSAEPs are also similar to those we previously reported in cats infected with the feline immunodeficiency virus (40) and those found by others in humans with HIV (41, 42). The delays in peak latencies of the later waves of the BSAEPs and AEPs complex are similar to our findings in chronically infected monkeys (22). Although the exact correspondence between the waveforms measured here and those studied in humans remains to be determined, later waves in the BSAEP likely represent activity of neurons in the upper brainstem, whereas the AEP waves are cortically generated. We find that these sensory-evoked potentials can be used as sensitive probes for alterations in neuronal circuitry, indicating perturbations in CNS neuronal function and activity. Because the electrophysiological abnormalities that develop after SIV infection affect the latencies of specific waves, and not the complex of potentials in general, it is likely that the virus, or a virus-induced cascade, selectively affects certain regions and/or functions of CNS neurons.
The detection of early and consistent changes in physiologic parameters allows the addition of important analyses to future antiviral or neuropharmacological treatment studies. Although the physiologic substrates involved in HIV-induced cognitive and motor abnormalities are unknown, we have identified distinct reproducible signs of physiological dysfunction in SIV-infected monkeys relevant to cognitive and motor function. These measures now can be used as parameters to examine both etiologic and therapeutic questions. For example, the relationship between both systemic and CNS viral load to the development of these abnormalities, to their potential reversibility with antiviral treatment, and to the time course with which they may return after treatment failure or withdrawal is of particular interest to therapeutic regimen choice and timing of drug administration in the current era of antiretroviral therapy.
The interesting observations that drugs such as 3′-azido-3′-deoxythymidine (Zidovudine, ZDV, AZT) and diadenosine may temporarily reverse HIV-related dementia in infected individuals (43, 44) are consistent with the idea that neurons, at least early, are not irreversibly damaged by a virus-induced pathological cascade. These findings also suggest alteration in neuronal function may be caused by a continuous, but reversible, release of neuromodulatory agents. This evidence suggests that the early forms of HIV-induced CNS damage may be amenable to pharmacotherapy that could obviate these “toxic” factors and prevent the chronic consequence of CNS dysfunction.
Acknowledgments
We thank Debbie Watry and Michelle Zandonatti for technical support, Dr. John Polich for advice on statistical analyses, and Drs. Floyd Bloom and Lisa Gold for contributions during the work and comments on the manuscript. This work was supported by grants from the National Institute of Mental Health (MH 55836 and MH 47680). This is manuscript No. 11145-NP from The Scripps Research Institute.
ABBREVIATIONS
- AEPs
auditory-evoked potentials
- BSAEPs
brainstem AEPs
- CNS
central nervous system
- CSF
cerebrospinal fluid
- EEG
electroencephalogram
- SIV
simian immunodeficiency virus
- p.i.
postinoculation
References
- 1.Chun T-W, Carruth L, Finzi D, Shen X, DiGiuseppe J A, Taylor H, Hermankova M, Chadwick K, Margolick J, Quinn T C, et al. Nature (London) 1997;387:183–188. doi: 10.1038/387183a0. [DOI] [PubMed] [Google Scholar]
- 2.Cavert W, Notermans D W, Staskus K, Wietgrefe S W, Zupancic M, Gebhard K, Henry K, Zhang Z-Q, Mills R, McDade H, et al. Science. 1997;276:960–964. doi: 10.1126/science.276.5314.960. [DOI] [PubMed] [Google Scholar]
- 3.Perelson A S, Essunger P, Cao Y, Vesanen M, Hurley A, Saksela K, Markowitz M, Ho D D. Nature (London) 1997;387:188–190. doi: 10.1038/387188a0. [DOI] [PubMed] [Google Scholar]
- 4.Chakrabarti L, Hurtrel M, Maire M-A, Vazeux R, Dormont D, Montagnier L, Hurtrel B. Am J Pathol. 1991;139:1273–1280. [PMC free article] [PubMed] [Google Scholar]
- 5.Lackner A A, Vogel P, Ramos R A, Kluge J D, Marthas M. Am J Pathol. 1994;145:428–439. [PMC free article] [PubMed] [Google Scholar]
- 6.Sharer L R, Michaels J, Murphey-Corb M, Hu F-S, Kuebler D J, Martin L N, Baskin G B. J Med Primatol. 1991;20:211–217. [PubMed] [Google Scholar]
- 7.Davis L E, Hjelle B L, Miller V E, Palmer D L, Llewellyn A L, Merlin T L, Young S A, Mills R G, Wachsman W, Wiley C A. Neurology. 1992;42:1736–1739. doi: 10.1212/wnl.42.9.1736. [DOI] [PubMed] [Google Scholar]
- 8.Orenstein J M, Fox C, Wahl S M. Science. 1997;270:1197–1199. [Google Scholar]
- 9.Kolson D L, Lavi E, Gonzalez-Scarano F. Adv Virus Res. 1998;50:1–47. doi: 10.1016/s0065-3527(08)60804-0. [DOI] [PubMed] [Google Scholar]
- 10.Darko D F, Miller J C, Gallen C, White J, Koziol J, Brown S J, Hayduk R, Atkinson J H, Assmus J, Munnell D T, et al. Proc Natl Acad Sci USA. 1995;92:12080–12084. doi: 10.1073/pnas.92.26.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Terstegge K, Henkes H, Scheuler W, Hansen M L, Ruf B, Kubicki S. Sleep. 1993;16:137–145. doi: 10.1093/sleep/16.2.137. [DOI] [PubMed] [Google Scholar]
- 12.Sacktor N, Bacellar H, Hoover D, Nance-Sproson T, Selnes O, Miller E, Dal Pan G, Kleeberger C, Brown A, Saah A, McArthur J. J Neurovirol. 1996;2:404–410. doi: 10.3109/13550289609146906. [DOI] [PubMed] [Google Scholar]
- 13.White J L, Darko D F, Brown S J, Miller J C, Hayduk R, Kelly T, Mitler M M. AIDS. 1995;9:1043–1050. doi: 10.1097/00002030-199509000-00009. [DOI] [PubMed] [Google Scholar]
- 14.Iragui V J, Kalmijn J, Thal L J, Grant I. Electroencephalogr Clin Neurophysiol. 1994;92:1–10. doi: 10.1016/0168-5597(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 15.Schmitt B, Seeger J, Jacobi G. Clin Electroencephalogr. 1992;23:111–117. doi: 10.1177/155005949202300304. [DOI] [PubMed] [Google Scholar]
- 16.Navia B A, Jordan B D, Price R W. Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 17.Smith C. Behav Brain Res. 1995;69:137–145. doi: 10.1016/0166-4328(95)00024-n. [DOI] [PubMed] [Google Scholar]
- 18.McCann U D, Penetar D M, Shaham Y, Thorne D R, Gillin J C, Sing H C, Thomas M A, Belenky G. Biol Psychiatry. 1992;31:1082–1097. doi: 10.1016/0006-3223(92)90153-q. [DOI] [PubMed] [Google Scholar]
- 19.Desrosiers R C. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- 20.Kindt T J, Hirsch V M, Johnson P R, Sawasdikosol S. Adv Immunol. 1992;52:425–473. doi: 10.1016/s0065-2776(08)60880-9. [DOI] [PubMed] [Google Scholar]
- 21.Murray E A, Rausch D M, Lendvay J, Sharer L R, Eiden L E. Science. 1992;255:1246–1249. doi: 10.1126/science.1546323. [DOI] [PubMed] [Google Scholar]
- 22.Prospero-Garcia O, Gold L H, Fox H S, Polis I, Koob G F, Bloom F E, Henriksen S J. Proc Natl Acad Sci USA. 1996;93:14158–14163. doi: 10.1073/pnas.93.24.14158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gold L H, Fox H S, Henriksen S J, Buchmeier M J, Weed M R, Taffe M A, Huitron-Resendez S, Horn T F W, Bloom F E. J Med Primatol. 1998;27:104–112. doi: 10.1111/j.1600-0684.1998.tb00234.x. [DOI] [PubMed] [Google Scholar]
- 24.Lane T E, Buchmeier M J, Watry D D, Jakubowski D B, Fox H S. Virology. 1995;212:458–465. doi: 10.1006/viro.1995.1503. [DOI] [PubMed] [Google Scholar]
- 25.Strelow L I, Watry D D, Fox H S, Nelson J A. J Neurovirol. 1998;4:269–280. doi: 10.3109/13550289809114528. [DOI] [PubMed] [Google Scholar]
- 26.Watry D, Lane T E, Streb M, Fox H S. Am J Pathol. 1995;146:914–923. [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson E L, Demaria-Pesce V H, Fuller C A. Am J Physiol. 1993;265:R781–R785. doi: 10.1152/ajpregu.1993.265.4.R781. [DOI] [PubMed] [Google Scholar]
- 28.Nowak M A, Lloyd A L, Vasquez G M, Wiltrout T A, Wahl L M, Bischofberger N, Williams J, Kinter A, Fauci A S, Hirsch V M, Lifson J D. J Virol. 1997;71:7518–7525. doi: 10.1128/jvi.71.10.7518-7525.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Petito C K, Cash K S. Ann Neurol. 1992;32:658–666. doi: 10.1002/ana.410320509. [DOI] [PubMed] [Google Scholar]
- 30.Singer E, Syndulko K, Fahy-Chandon B, Shapshak P, Resnick L, Schmid P, Conrad A, Tourtellotte W. AIDS. 1994;8:197–204. doi: 10.1097/00002030-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 31.Smith M, Sutjipto S, Lackner A. AIDS Res Hum Retroviruses. 1994;10:81–89. doi: 10.1089/aid.1994.10.81. [DOI] [PubMed] [Google Scholar]
- 32.Lipton S A. Curr Opin Neurol. 1997;10:247–253. doi: 10.1097/00019052-199706000-00014. [DOI] [PubMed] [Google Scholar]
- 33.Lipton S A, Gendelman H E. N Engl J Med. 1995;332:934–940. doi: 10.1056/NEJM199504063321407. [DOI] [PubMed] [Google Scholar]
- 34.Bollinger R C, Brookmeyer R S, Mehendale S M, Paranjape R S, Shepherd M E, Gadkari D A, Quinn T C. J Am Med Assoc. 1997;278:2085–2089. [PubMed] [Google Scholar]
- 35.Gold S, Cahani M, Sohmer H, Horowitz M, Shahar A. Electroencephalogr Clin Neurophysiol. 1985;60:146–153. doi: 10.1016/0013-4694(85)90021-5. [DOI] [PubMed] [Google Scholar]
- 36.Kleuger M J, Kozak W, Leon L R, Soszynski D, Conn C A. Neuroimmunomodulation. 1995;2:216–223. doi: 10.1159/000097199. [DOI] [PubMed] [Google Scholar]
- 37.Krueger J M, Takahashi S, Kapas L, Bredow S, Roky R, Fang J, Floyd R, Renegar K B, Guha-Thakurta N, Novitsky S, Obal S. Adv Neuroimmunol. 1995;5:171–188. doi: 10.1016/0960-5428(95)00007-o. [DOI] [PubMed] [Google Scholar]
- 38.Andersen P, Moser E I. Hippocampus. 1995;5:491–498. doi: 10.1002/hipo.450050602. [DOI] [PubMed] [Google Scholar]
- 39.Rausch D M, Heyes M P, Murray E A, Lendvay J, Sharer L R, Ward J M, Rehm S, Nohr D, Weihe E, Eiden L E. J Neuropathol Exp Neurol. 1994;53:165–175. doi: 10.1097/00005072-199403000-00008. [DOI] [PubMed] [Google Scholar]
- 40.Phillips T R, Prospero-Garcia O, Puaoi D L, Lerner D L, Fox H S, Olmsted R A, Bloom F E, Henriksen S J, Elder J H. J Gen Virol. 1994;75:979–987. doi: 10.1099/0022-1317-75-5-979. [DOI] [PubMed] [Google Scholar]
- 41.Pagano M A, Cahn P E, Garau M L, Mangone C A, Figini H A, Yorio A A, Dellepiane M C, Amores M G, Perez H M, Casiro A D. Arch Neurol. 1992;49:166–169. doi: 10.1001/archneur.1992.00530260068022. [DOI] [PubMed] [Google Scholar]
- 42.Somma-Mauvais H, Farnarier G. Neurophysiol Clin. 1992;22:369–384. doi: 10.1016/s0987-7053(05)80095-4. [DOI] [PubMed] [Google Scholar]
- 43.Pizzo P A, Eddy J, Falloon J, Balis F M, Murphy R F, Moss H, Wolters P, Brouwers P, Jarosinki P, Rubin M, et al. N Engl J Med. 1988;319:889–896. doi: 10.1056/NEJM198810063191401. [DOI] [PubMed] [Google Scholar]
- 44.Brouwers P, Hendricks M, Lietzau J A, J M, P, Mitsuya H, Broder S, Yarchoan R. AIDS. 1997;11:59–66. doi: 10.1097/00002030-199701000-00009. [DOI] [PubMed] [Google Scholar]