Abstract
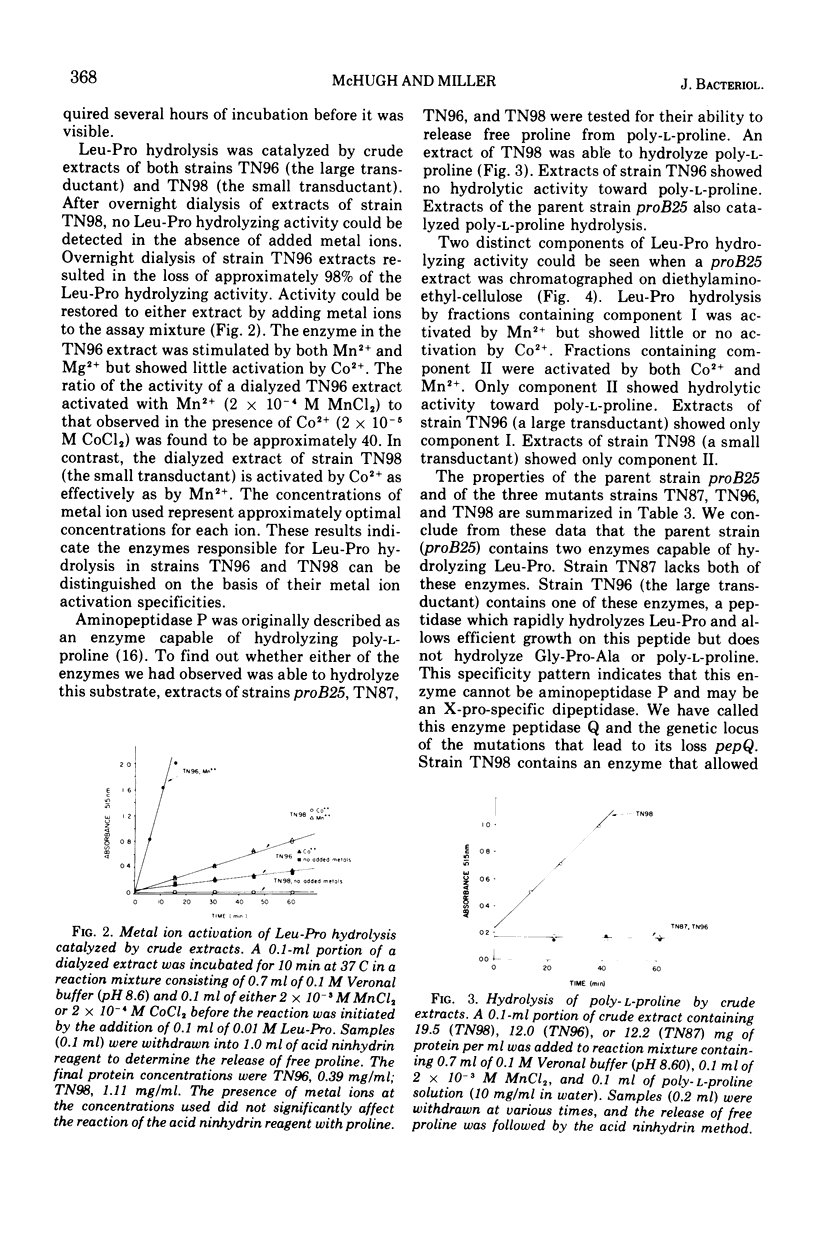
The proline requirement of Salmonella typhimurium strain proB25 can be satisfied by either of the peptides Leu-Pro or Gly-Pro-Ala. A mutant derivative of strain proB25 isolated by penicillin selection in medium containing Leu-Pro as proline source fails to use either Leu-Pro or Gly-Pro-Ala as a source of proline. This strain is a double mutant that lacks two aminoacyl-proline-specific peptidases. One of these enzymes (peptidase Q) catalyzes the rapid hydrolysis of Leu-Pro but does not hydrolyze Gly-Pro-Ala or poly-l-proline. Mutations at a site (pepQ) near metE lead to loss of this activity. The other peptidase (peptidase P) catalyzes the hydrolysis of Gly-Pro-Ala and poly-l-proline but is only weakly active with Leu-Pro as substrate. This enzyme is similar to aminopeptidase P previously described in Escherichia coli (16). Mutations at a locus (pepP) near serA lead to loss of this enzyme.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- DAVIS B. J. DISC ELECTROPHORESIS. II. METHOD AND APPLICATION TO HUMAN SERUM PROTEINS. Ann N Y Acad Sci. 1964 Dec 28;121:404–427. doi: 10.1111/j.1749-6632.1964.tb14213.x. [DOI] [PubMed] [Google Scholar]
- Gutnick D., Calvo J. M., Klopotowski T., Ames B. N. Compounds which serve as the sole source of carbon or nitrogen for Salmonella typhimurium LT-2. J Bacteriol. 1969 Oct;100(1):215–219. doi: 10.1128/jb.100.1.215-219.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Itikawa H., Demerec M. Salmonella typhimurium proline mutants. J Bacteriol. 1968 Mar;95(3):1189–1190. doi: 10.1128/jb.95.3.1189-1190.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lewis W. H., Harris H. Human red cell peptidases. Nature. 1967 Jul 22;215(5099):351–355. doi: 10.1038/215351a0. [DOI] [PubMed] [Google Scholar]
- Miller C. G., Mackinnon K. Peptidase mutants of Salmonella typhimurium. J Bacteriol. 1974 Oct;120(1):355–363. doi: 10.1128/jb.120.1.355-363.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pine M. J. Turnover of intracellular proteins. Annu Rev Microbiol. 1972;26:103–126. doi: 10.1146/annurev.mi.26.100172.000535. [DOI] [PubMed] [Google Scholar]
- Roth J. R., Sanderson K. E. Orientation of the isoleucine-valine genes in the Salmonella typhimurium linkage map. Genetics. 1966 May;53(5):971–976. doi: 10.1093/genetics/53.5.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SARID S., BERGER A., KATCHALSKI E. Proline iminopeptidase. J Biol Chem. 1959 Jul;234(7):1740–1746. [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TROLL W., LINDSLEY J. A photometric method for the determination of proline. J Biol Chem. 1955 Aug;215(2):655–660. [PubMed] [Google Scholar]
- VOGEL H. J., BONNER D. M. Acetylornithinase of Escherichia coli: partial purification and some properties. J Biol Chem. 1956 Jan;218(1):97–106. [PubMed] [Google Scholar]




