Abstract
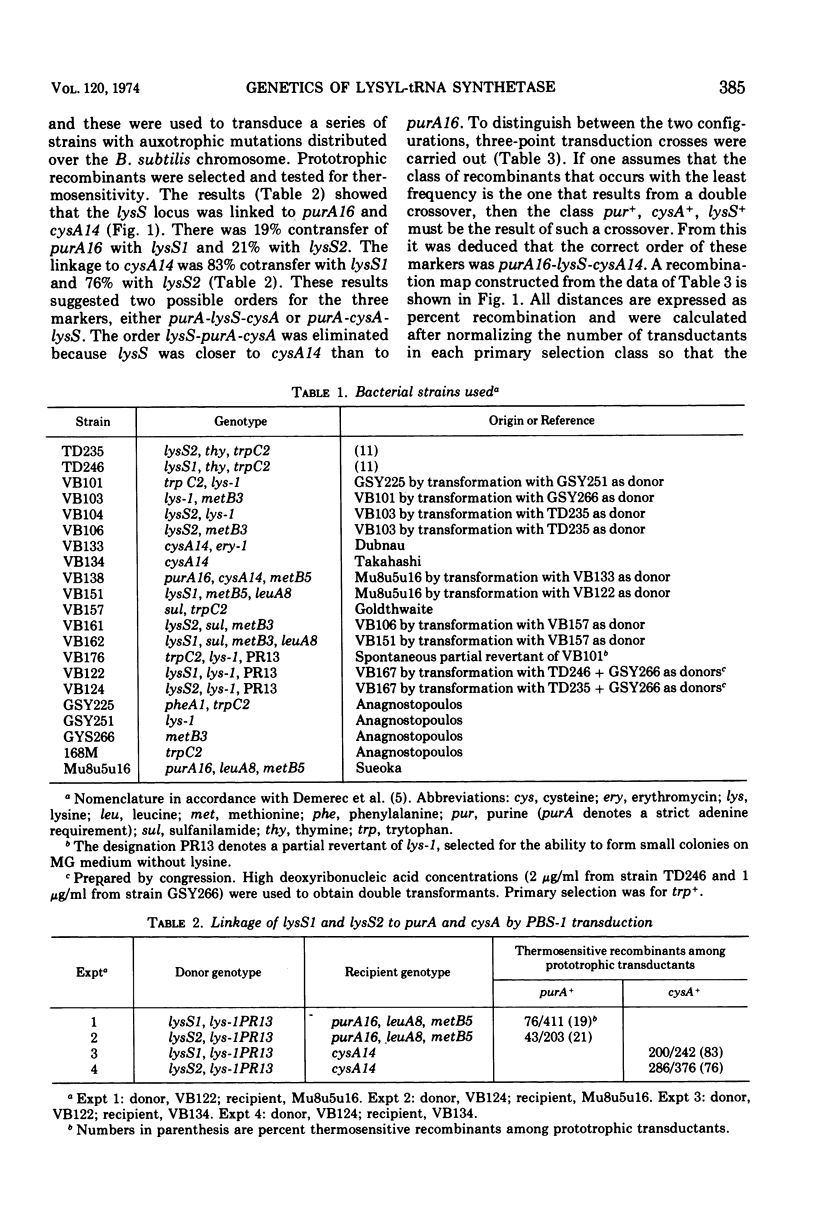
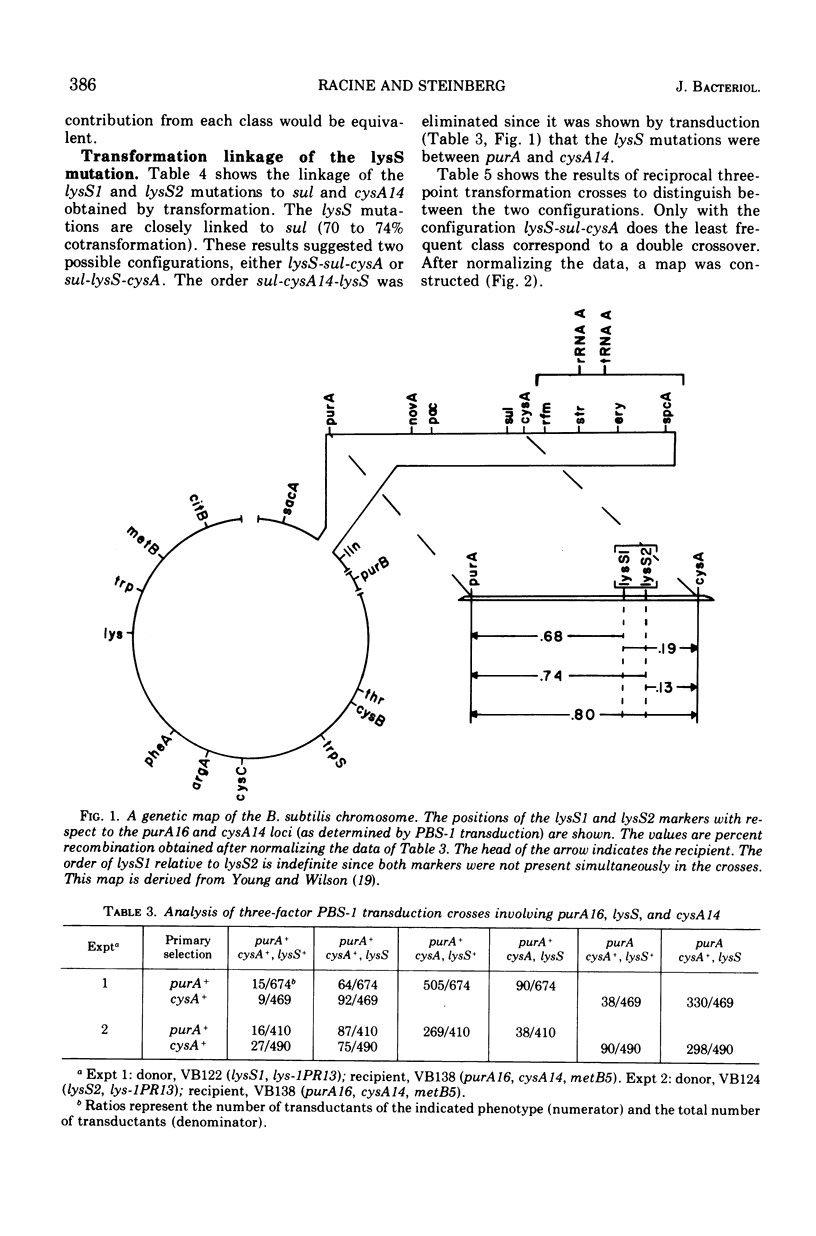
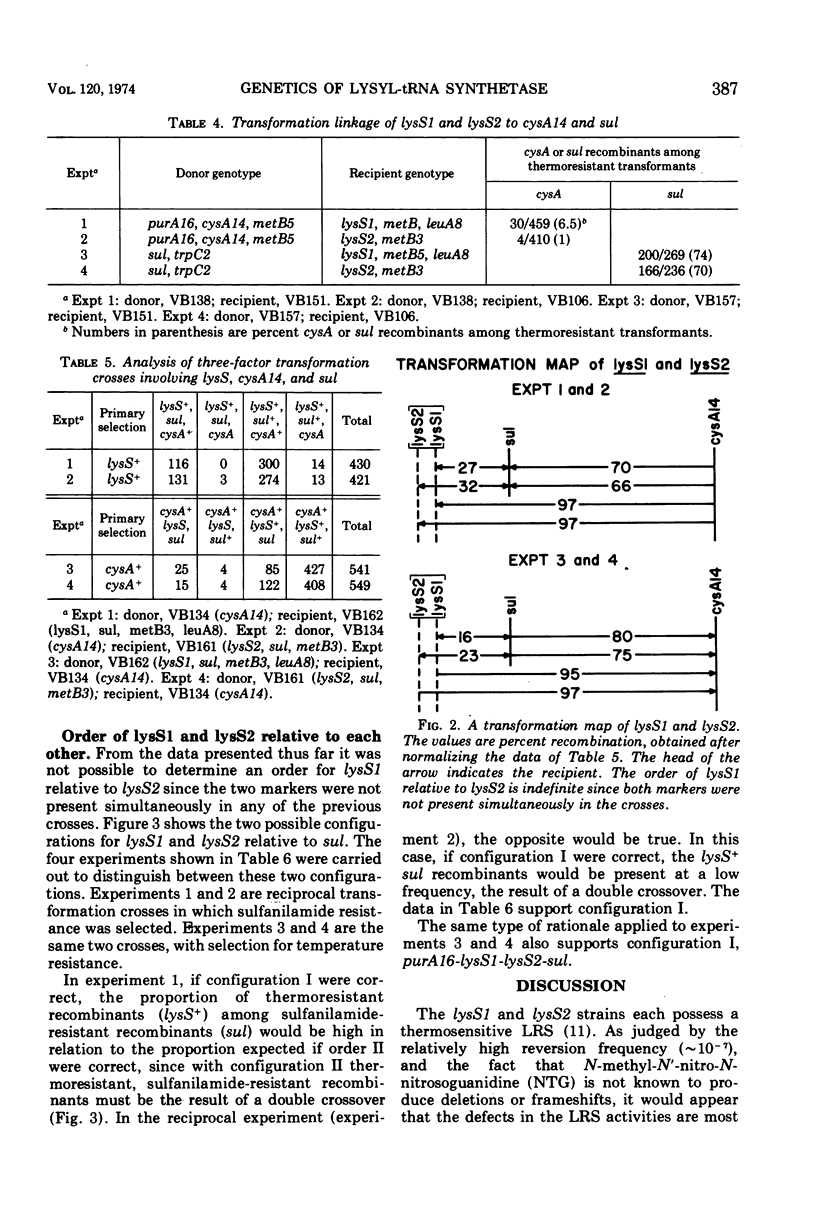
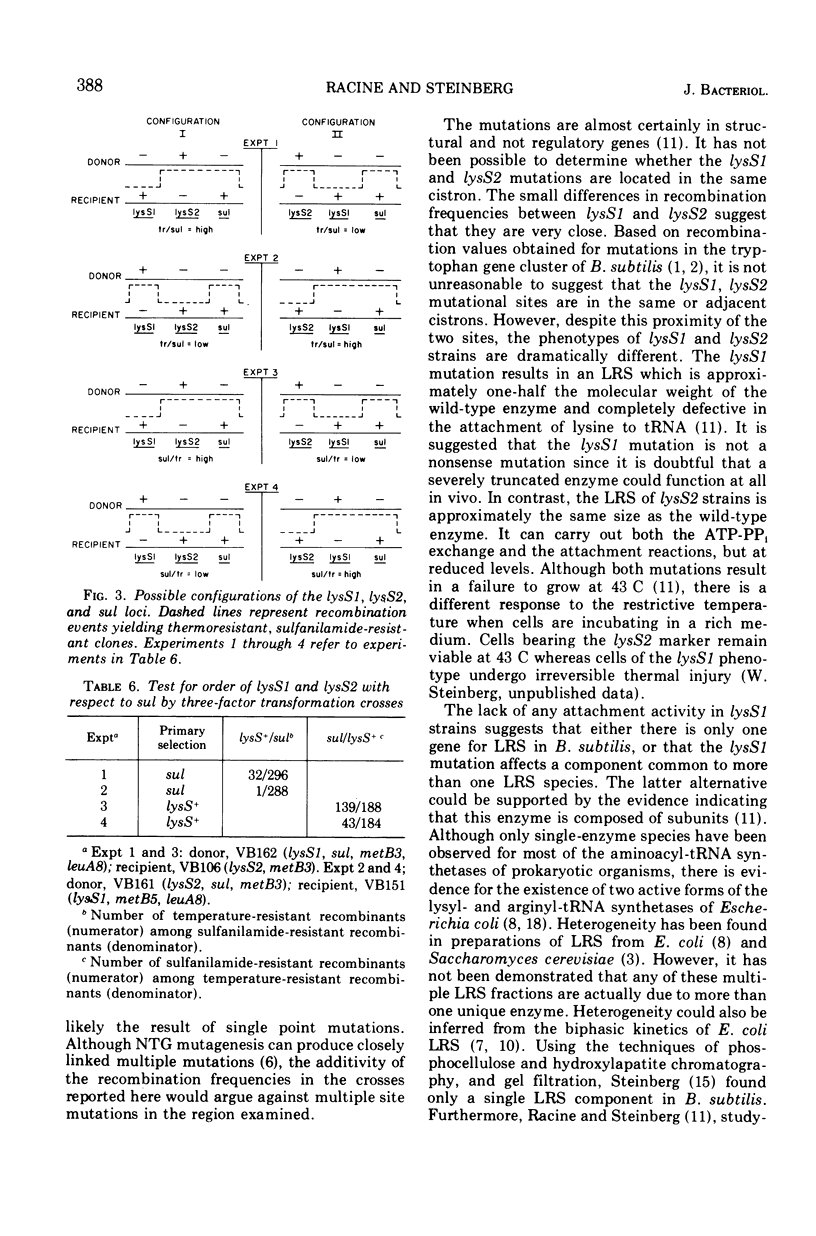
Two mutations (lysS1 and lysS2), each independently resulting in a thermosensitive, lysyl-transfer RNA synthetase (l-lysine: tRNA ligase [adenosine 5′-monophosphate] EC 6.1.1.6), have been mapped on the Bacillus subtilis chromosome between purA16 (adenine requirement) and sul (sulfanilamide resistance). They are linked by transformation with sul (70 to 74% cotransfer) in the order purA16-lysS1-lysS2-sul. The mutant loci are either in the same gene or in two closely linked genes. They are not linked to the tryptophanyl-tRNA synthetase structural gene or to the lys-1 locus.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anagnostopoulos C., Crawford I. P. Le groupe des gènes régissant la biosynthèse du tryptophane chez Bacillus subtilis. C R Acad Sci Hebd Seances Acad Sci D. 1967 Jul 3;265(1):93–96. [PubMed] [Google Scholar]
- Carlton B. C. Fine-structure mapping by transformation in the tryptophan region of Bacillus subtilis. J Bacteriol. 1966 May;91(5):1795–1803. doi: 10.1128/jb.91.5.1795-1803.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chlumecká V., Von Tigerstrom M., D'Obrenan P., Smith C. J. Purification and properties of lysyl transfer ribonucleic acid synthetase from bakers' yeast. J Biol Chem. 1969 Oct 25;244(20):5481–5488. [PubMed] [Google Scholar]
- Clarke S. J., Low B., Konigsberg W. H. Close linkage of the genes serC (for phosphohydroxy pyruvate transaminase) and serS (for seryl-transfer ribonucleic acid synthetase) in Escherichia coli K-12. J Bacteriol. 1973 Mar;113(3):1091–1095. doi: 10.1128/jb.113.3.1091-1095.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demerec M., Adelberg E. A., Clark A. J., Hartman P. E. A proposal for a uniform nomenclature in bacterial genetics. Genetics. 1966 Jul;54(1):61–76. doi: 10.1093/genetics/54.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerola N., Ingraham J. L., Cerdá-Olmedo E. Induction of closely linked multiple mutations by nitrosoguanidine. Nat New Biol. 1971 Mar 24;230(12):122–125. doi: 10.1038/newbio230122a0. [DOI] [PubMed] [Google Scholar]
- Hele P., Barber R. Lysyl tRNA synthetase of Escherichia coli B: formation and reactions of ATP-enzyme and lysyl-AMP-enzyme complexes. Biochim Biophys Acta. 1972 Jan 20;258(1):319–331. doi: 10.1016/0005-2744(72)90989-8. [DOI] [PubMed] [Google Scholar]
- Kisselev L. L., Baturina I. D. Two enzymatically active forms of lysyl-tRNA synthetase from E. coli B. FEBS Lett. 1972 May 1;22(2):231–234. doi: 10.1016/0014-5793(72)80052-8. [DOI] [PubMed] [Google Scholar]
- LEDERBERG J., LEDERBERG E. M. Replica plating and indirect selection of bacterial mutants. J Bacteriol. 1952 Mar;63(3):399–406. doi: 10.1128/jb.63.3.399-406.1952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall R. D., Zamecnik P. C. Aspects of the kinetic properties of lysyl-tRNA synthetase from Escherichia coli, strain B. Biochim Biophys Acta. 1970 Feb 11;198(2):376–385. doi: 10.1016/0005-2744(70)90070-7. [DOI] [PubMed] [Google Scholar]
- Racine F. M., Steinberg W. Defects of two temperature-sensitive lysyl-transfer ribonucleic acid synthetase mutants of Bacillus subtilis. J Bacteriol. 1974 Oct;120(1):372–383. doi: 10.1128/jb.120.1.372-383.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson K. E. Linkage map of Salmonella typhimurium, edition IV. Bacteriol Rev. 1972 Dec;36(4):558–586. doi: 10.1128/br.36.4.558-586.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spizizen J. TRANSFORMATION OF BIOCHEMICALLY DEFICIENT STRAINS OF BACILLUS SUBTILIS BY DEOXYRIBONUCLEATE. Proc Natl Acad Sci U S A. 1958 Oct 15;44(10):1072–1078. doi: 10.1073/pnas.44.10.1072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W., Anagnostopoulos C. Biochemical and genetic characterization of a temperature-sensitive, tryptophanyl-transfer ribonucleic acid synthetase mutant of Bacillus subtilis. J Bacteriol. 1971 Jan;105(1):6–19. doi: 10.1128/jb.105.1.6-19.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W. Properties and developmental roles of the lysyl- and tryptophanyl-transfer ribonucleic acid synthetases of Bacillus subtilis: common genetic origin of the corresponding spore and vegetative enzymes. J Bacteriol. 1974 Apr;118(1):70–82. doi: 10.1128/jb.118.1.70-82.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg W. Temperature-induced derepression of tryptophan biosynthesis in a tryptophanyl-transfer ribonucleic acid synthetase mutant of Bacillus subtilis. J Bacteriol. 1974 Mar;117(3):1023–1034. doi: 10.1128/jb.117.3.1023-1034.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor A. L., Trotter C. D. Linkage map of Escherichia coli strain K-12. Bacteriol Rev. 1972 Dec;36(4):504–524. doi: 10.1128/br.36.4.504-524.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yem D. W., Williams L. S. Evidence for the existence of two arginyl-transfer ribonucleic acid synthetase activities in Escherichia coli. J Bacteriol. 1973 Feb;113(2):891–894. doi: 10.1128/jb.113.2.891-894.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]



