Abstract
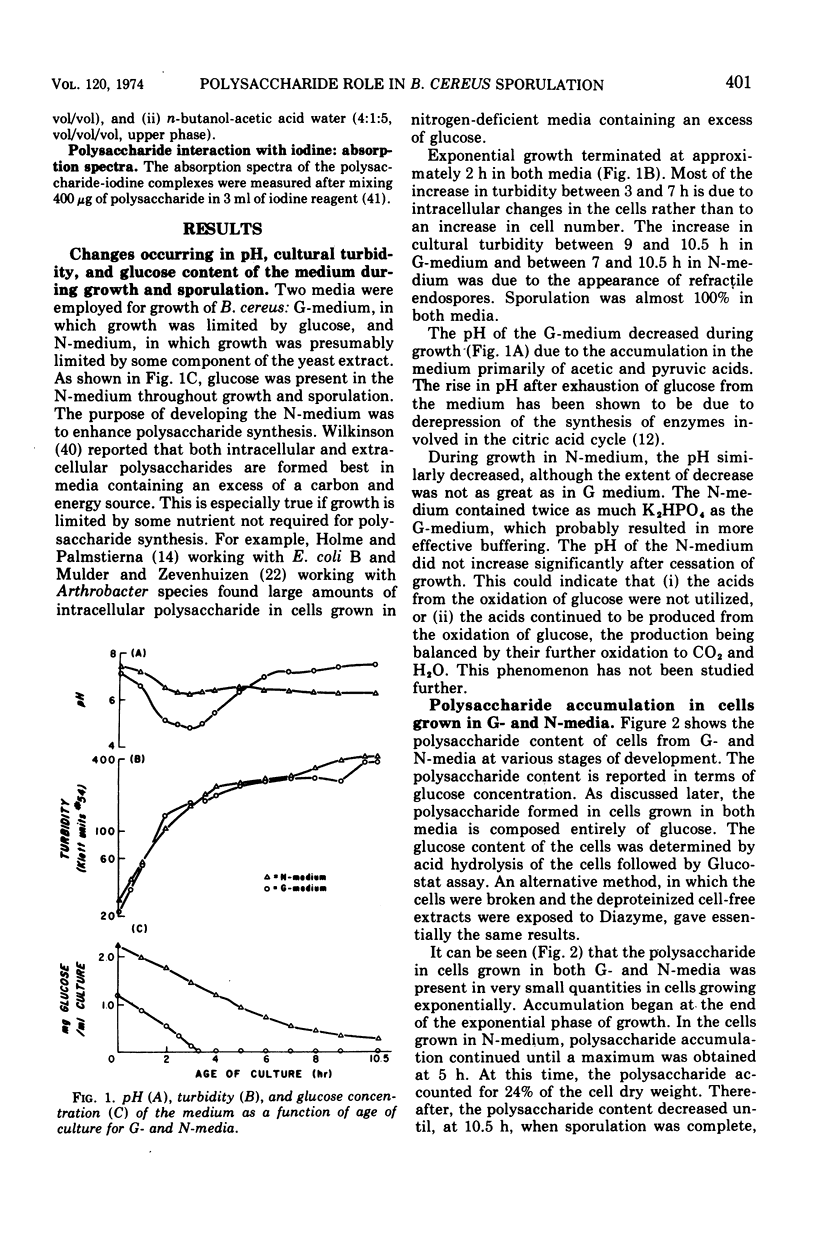
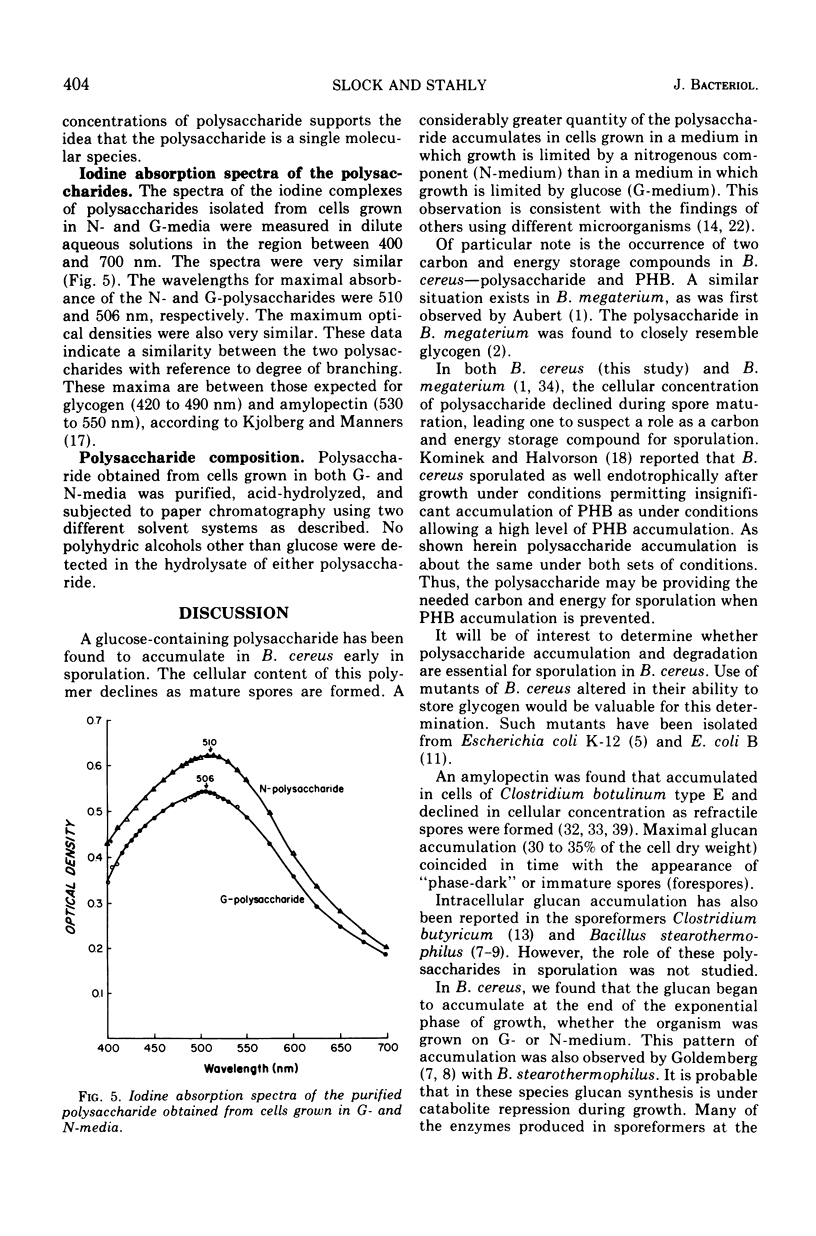
An intracellular, glucose-containing polysaccharide accumulates in Bacillus cereus early in sporulation and is degraded at the time of spore maturation. This pattern of accumulation and degradation occurred when growth was limited by glucose or a component of yeast extract. These data suggest that the polysaccharide may be serving as a carbon and energy storage compound for sporulation. A somewhat similar pattern of accumulation and degradation of poly-β-hydroxybutyric acid (PHB) was shown earlier by Kominek and Halvorson (1965) to occur in Bacillus cereus. When cells were grown in a medium buffered strongly at pH 7.4, however, very little accumulation of PHB occurred. We have found that polysaccharide accumulates in cells grown in both the strong and weakly buffered media. Perhaps polysaccharide is the major carbon and energy storage compound when cells are grown under conditions preventing significant accumulation of PHB. The lack of polysaccharide accumulation during the exponential phase of growth may be an indication that the needed biosynthetic enzymes are controlled by catabolite repression during growth. The polysaccharide was purified and found to consist of glucose. The iodine absorption spectrum suggests a degree of branching between that of glycogen and amylopectin.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AUBERT J. P. Etude biochimique du rendement matériel de croissance d'une bactérie aérobie: Bacillus megatherium. Ann Inst Pasteur (Paris) 1951 Jun;80(6):644–658. [PubMed] [Google Scholar]
- BARRY C., GAVARD R., MILHAUD G., AUBERT J. P. Etude du glycogène extrait de Bacillus megatherium. Ann Inst Pasteur (Paris) 1953 Mar;84(3):605–613. [PubMed] [Google Scholar]
- Brewer S. J., Berkeley R. C. Control of the production of exo-beta-N-acetylglucosaminidase by Bacillus subtilis B. Biochem J. 1973 May;134(1):271–281. doi: 10.1042/bj1340271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damotte M., Cattanéo J., Sigal N., Puig J. Mutants of Escherichia coli K 12 altered in their ability to store glycogen. Biochem Biophys Res Commun. 1968 Sep 30;32(6):916–920. doi: 10.1016/0006-291x(68)90114-9. [DOI] [PubMed] [Google Scholar]
- Goldemberg S. H., Algranati I. D. [Biosynthesis of an alpha-1,4-glucan by extracts of a thermophilic bacterium]. Biochim Biophys Acta. 1969 Feb 18;177(1):166–168. doi: 10.1016/0304-4165(69)90082-8. [DOI] [PubMed] [Google Scholar]
- Goldemberg S. H. Glucan biosynthesis in Bacillus stearothermophilus. I. Properties of the polysaccharide. Arch Biochem Biophys. 1972 Mar;149(1):252–258. doi: 10.1016/0003-9861(72)90320-7. [DOI] [PubMed] [Google Scholar]
- Govons S., Vinopal R., Ingraham J., Preiss J. Isolation of mutants of Escherichia coli B altered in their ability to synthesize glycogen. J Bacteriol. 1969 Feb;97(2):970–972. doi: 10.1128/jb.97.2.970-972.1969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANSON R. S., SRINIVASAN V. R., HALVORSON H. O. BIOCHEMISTRY OF SPORULATION. II. ENZYMATIC CHANGES DURING SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Jul;86:45–50. doi: 10.1128/jb.86.1.45-50.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. A., Fusaro R. M. The quantitative enzymic determination of animal liver glycogen. Anal Biochem. 1966 Apr;15(1):140–149. doi: 10.1016/0003-2697(66)90256-9. [DOI] [PubMed] [Google Scholar]
- Kominek L. A., Halvorson H. O. Metabolism of poly-beta-hydroxybutyrate and acetoin in Bacillus cereus. J Bacteriol. 1965 Nov;90(5):1251–1259. doi: 10.1128/jb.90.5.1251-1259.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MONTGOMERY R. Determination glycogen. Arch Biochem Biophys. 1957 Apr;67(2):378–386. doi: 10.1016/0003-9861(57)90292-8. [DOI] [PubMed] [Google Scholar]
- Mulder E. G., Zevenhuizen L. P. Coryneform bacteria of the Arthrobacter type and their reserve material. Arch Mikrobiol. 1967;59(1):345–354. doi: 10.1007/BF00406348. [DOI] [PubMed] [Google Scholar]
- NAKATA H. M. EFFECT OF PH ON INTERMEDIATES PRODUCED DURING GROWTH AND SPORULATION OF BACILLUS CEREUS. J Bacteriol. 1963 Sep;86:577–581. doi: 10.1128/jb.86.3.577-581.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NAKATA H. M., HALVORSON H. O. Biochemical changes occurring during growth and sporulation of Bacillus cereus. J Bacteriol. 1960 Dec;80:801–810. doi: 10.1128/jb.80.6.801-810.1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renner E. D., Bernlohr R. W. Characterization and regulation of pyruvate carboxylase of Bacillus licheniformis. J Bacteriol. 1972 Feb;109(2):764–772. doi: 10.1128/jb.109.2.764-772.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouser G., Siakotos A. N., Fleischer S. Quantitative analysis of phospholipids by thin-layer chromatography and phosphorus analysis of spots. Lipids. 1966 Jan;1(1):85–86. doi: 10.1007/BF02668129. [DOI] [PubMed] [Google Scholar]
- STEWART B. T., HALVORSON H. O. Studies on the spores of aerobic bacteria. I. The occurrence of alanine racemase. J Bacteriol. 1953 Feb;65(2):160–166. doi: 10.1128/jb.65.2.160-166.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slepecky R. A., Law J. H. SYNTHESIS AND DEGRADATION OF POLY-beta-HYDROXYBUTYRIC ACID IN CONNECTION WITH SPORULATION OF BACILLUS MEGATERIUM. J Bacteriol. 1961 Jul;82(1):37–42. doi: 10.1128/jb.82.1.37-42.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strasdine G. A. Amylopectin accumulation in Clostridium botulinum type E. Can J Microbiol. 1968 Oct;14(10):1059–1062. doi: 10.1139/m68-178. [DOI] [PubMed] [Google Scholar]
- Strasdine G. A. The role of intracellular glucan in endogenous fermentation and spore maturation in Clostridium botulinum type E. Can J Microbiol. 1972 Feb;18(2):211–217. doi: 10.1139/m72-033. [DOI] [PubMed] [Google Scholar]
- TREVELYAN W. E., PROCTER D. P., HARRISON J. S. Detection of sugars on paper chromatograms. Nature. 1950 Sep 9;166(4219):444–445. doi: 10.1038/166444b0. [DOI] [PubMed] [Google Scholar]
- Van Handel E. Estimation of glycogen in small amounts of tissue. Anal Biochem. 1965 May;11(2):256–265. doi: 10.1016/0003-2697(65)90013-8. [DOI] [PubMed] [Google Scholar]
- Walters J. R., Stahly D. P. Modification of the valve of the French pressure cell. Appl Microbiol. 1968 Oct;16(10):1605–1605. doi: 10.1128/am.16.10.1605-.1968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whyte J. N., Strasdine G. A. An intracellular alpha-D-glucan from Clostridium botulinum, type E. Carbohydr Res. 1972 Dec;25(2):435–441. doi: 10.1016/s0008-6215(00)81655-9. [DOI] [PubMed] [Google Scholar]



