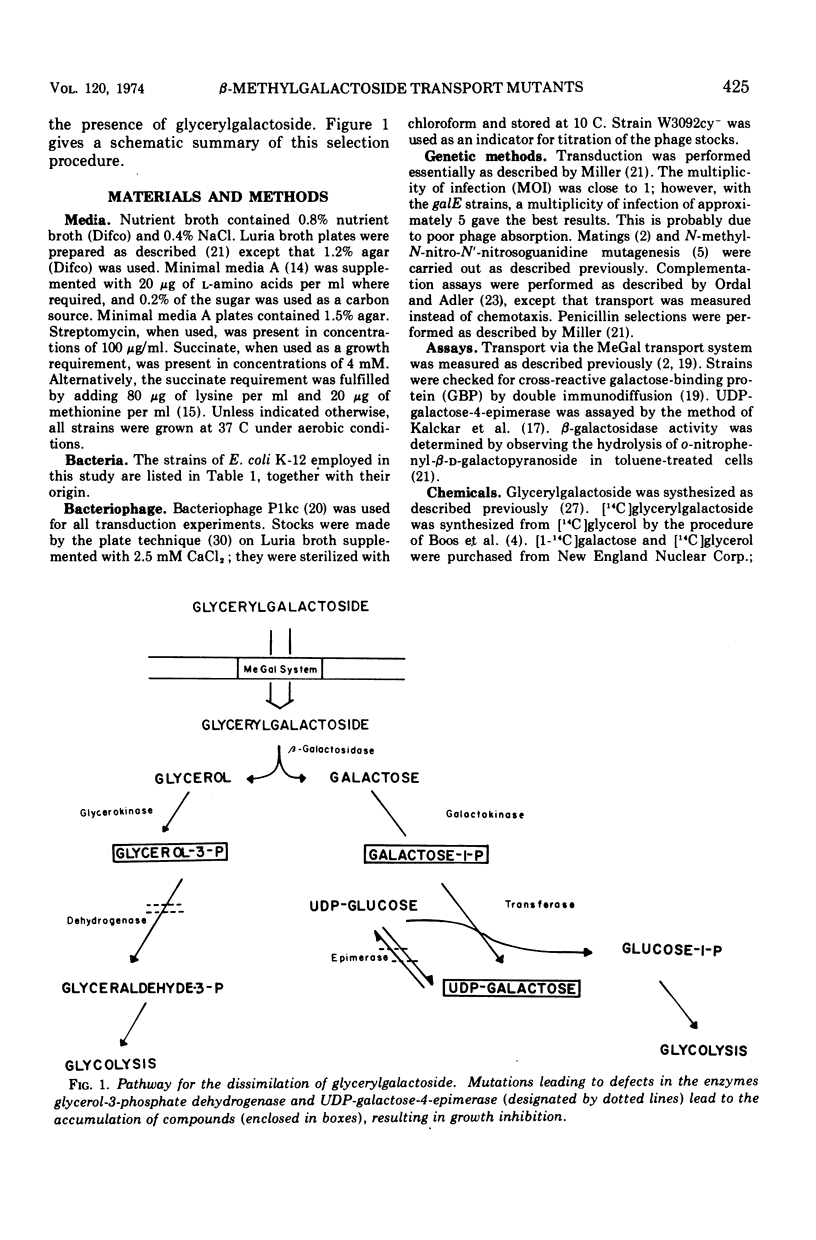
Abstract
A procedure has been devised that allows selection of mutants defective in the β-methylgalactoside transport system (mgl) of Escherichia coli. This procedure utilizes the compound 2R-glyceryl-β-d-galactopyranoside (glycerylgalactoside), which is known to be transported by only two transport system in E. coli, namely, the lactose and the β-methylgalactoside transport systems. Mutants lacking glycerol-3-phosphate dehydrogenase (glpD) are sensitive to glycerol. Similarly, mutants lacking uridine diphosphate-galactose-4-epimerase (galE) are sensitive to galactose. Glycerylgalactoside is an inducer of the lactose operon and also a substrate for β-galactosidase. Thus, a mgl+glpD galE lacY strain will not grow in the presence of glycerylgalactoside owing to accumulated glycerol-3-phosphate, galactose-1-phosphate, and uridine diphosphate-galactose. We have constructed such a strain and shown that mgl mutants can be obtained by selecting for those that grow in the presence of glycerylgalactoside.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Appleyard R K. Segregation of New Lysogenic Types during Growth of a Doubly Lysogenic Strain Derived from Escherichia Coli K12. Genetics. 1954 Jul;39(4):440–452. doi: 10.1093/genetics/39.4.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BURSTEIN C., COHN M., KEPES A., MONOD J. R OLE DU LACTOSE ET DE SES PRODUITS M'ETABOLIQUES DANS L'INDUCTION DE L'OP'ERON LACTOSE CHEZ ESCHERICHIA COLI. Biochim Biophys Acta. 1965 Apr 19;95:634–639. [PubMed] [Google Scholar]
- Boos W., Lengeler J., Hermann K. O., Unsöld H. J. The regulation of the beta-methylgalactoside transport system and of the galactose binding protein of Escherichia coli K12. Eur J Biochem. 1971 Apr 30;19(4):457–470. doi: 10.1111/j.1432-1033.1971.tb01336.x. [DOI] [PubMed] [Google Scholar]
- Boos W., Sarvas M. O. Close linkage between a galactose binding protein and the beta-methylgalactoside permease in Escherichia coli. Eur J Biochem. 1970 Apr;13(3):526–533. doi: 10.1111/j.1432-1033.1970.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Boos W. Structurally defective galactose-binding protein isolated from a mutant negative in the -methylgalactoside transport system of Escherichia coli. J Biol Chem. 1972 Sep 10;247(17):5414–5424. [PubMed] [Google Scholar]
- Cozzarelli N. R., Freedberg W. B., Lin E. C. Genetic control of L-alpha-glycerophosphate system in Escherichia coli. J Mol Biol. 1968 Feb 14;31(3):371–387. doi: 10.1016/0022-2836(68)90415-4. [DOI] [PubMed] [Google Scholar]
- Cozzarelli N. R., Koch J. P., Hayashi S., Lin E. C. Growth stasis by accumulated L-alpha-glycerophosphate in Escherichia coli. J Bacteriol. 1965 Nov;90(5):1325–1329. doi: 10.1128/jb.90.5.1325-1329.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Formation of protoplasts' in mutant strains of Salmonella induced by galactose. Nature. 1959 Apr 18;183(4668):1131–1132. doi: 10.1038/1831131a0. [DOI] [PubMed] [Google Scholar]
- FUKASAWA T., NIKAIDO H. Galactose-sensitive mutants of Salmonella. Nature. 1959 Oct 10;184(Suppl 15):1168–1169. doi: 10.1038/1841168a0. [DOI] [PubMed] [Google Scholar]
- Ferenci T., Kornberg H. L. Pathway of fructose utilization by Escherichia coli. FEBS Lett. 1971 Feb 19;13(2):127–130. doi: 10.1016/0014-5793(71)80216-8. [DOI] [PubMed] [Google Scholar]
- Gilbert W., Müller-Hill B. Isolation of the lac repressor. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1891–1898. doi: 10.1073/pnas.56.6.1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halpern Y. S., Even-Shoshan A. Properties of the glutamate transport system in Escherichia coli. J Bacteriol. 1967 Mar;93(3):1009–1016. doi: 10.1128/jb.93.3.1009-1016.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herbert A. A., Guest J. R. Studies with alpha-ketoglutarate dehydrogenase mutants of Escherichia coli. Mol Gen Genet. 1969 Oct 13;105(2):182–190. doi: 10.1007/BF00445687. [DOI] [PubMed] [Google Scholar]
- Kalckar H. M., Kurahashi K., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, I. DETERMINATION OF ENZYME ACTIVITIES. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1776–1786. doi: 10.1073/pnas.45.12.1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalckar H. M. The periplasmic galactose binding protein of Escherichia coli. Science. 1971 Nov 5;174(4009):557–565. doi: 10.1126/science.174.4009.557. [DOI] [PubMed] [Google Scholar]
- Kashket E. R., Wilson T. H. Isolation and properties of mutants of Escherichia coli with increased phosphorylations of thiomethyl-beta-galactoside. Biochim Biophys Acta. 1969;193(2):294–307. doi: 10.1016/0005-2736(69)90190-4. [DOI] [PubMed] [Google Scholar]
- LENNOX E. S. Transduction of linked genetic characters of the host by bacteriophage P1. Virology. 1955 Jul;1(2):190–206. doi: 10.1016/0042-6822(55)90016-7. [DOI] [PubMed] [Google Scholar]
- Ordal G. W., Adler J. Isolation and complementation of mutants in galactose taxis and transport. J Bacteriol. 1974 Feb;117(2):509–516. doi: 10.1128/jb.117.2.509-516.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapin A. M., Kalckar H. M., Alberico L. The metabolic basis for masking of receptor-sites on E. coli K-12 for C21, a lipopolysaccharide core-specific phage. Arch Biochem Biophys. 1968 Oct;128(1):95–105. doi: 10.1016/0003-9861(68)90011-8. [DOI] [PubMed] [Google Scholar]
- Reid K. G., Utech N. M., Holden J. T. Multiple transport components for dicarboxylic amino acids in Streptococcus faecalis. J Biol Chem. 1970 Oct 25;245(20):5261–5272. [PubMed] [Google Scholar]
- Rotman B., Ganesan A. K., Guzman R. Transport systems for galactose and galactosides in Escherichia coli. II. Substrate and inducer specificities. J Mol Biol. 1968 Sep 14;36(2):247–260. doi: 10.1016/0022-2836(68)90379-3. [DOI] [PubMed] [Google Scholar]
- SWANSTROM M., ADAMS M. H. Agar layer method for production of high titer phage stocks. Proc Soc Exp Biol Med. 1951 Nov;78(2):372–375. doi: 10.3181/00379727-78-19076. [DOI] [PubMed] [Google Scholar]
- Silhavy T. J., Boos W. A convenient synthesis of (2R)-glyceryl-beta-D-galactopyranoside. A substrate for beta-galactosidase, the lactose repressor, the galactose-binding protein, and the beta-methylgalactoside transport system. J Biol Chem. 1973 Sep 25;248(18):6571–6574. [PubMed] [Google Scholar]
- Wilson D. B. The regulation and properties of the galactose transport system in Escherichia coli K12. J Biol Chem. 1974 Jan 25;249(2):553–558. [PubMed] [Google Scholar]
- Wolfinbarger L., Jr, DeBusk A. G. The kinetics of L-aspartate transport in Neurospora crassa conidia. Biochim Biophys Acta. 1972 Dec 1;290(1):355–367. doi: 10.1016/0005-2736(72)90078-8. [DOI] [PubMed] [Google Scholar]
- Wu H. C., Boos W., Kalckar H. M. Role of the galactose transport system in the retention of intracellular galactose in Escherichia coli. J Mol Biol. 1969 Apr 14;41(1):109–120. doi: 10.1016/0022-2836(69)90129-6. [DOI] [PubMed] [Google Scholar]
- Yarmolinsky M. B., Wiesmeyer H., Kalckar H. M., Jordan E. HEREDITARY DEFECTS IN GALACTOSE METABOLISM IN ESCHERICHIA COLI MUTANTS, II. GALACTOSE-INDUCED SENSITIVITY. Proc Natl Acad Sci U S A. 1959 Dec;45(12):1786–1791. doi: 10.1073/pnas.45.12.1786. [DOI] [PMC free article] [PubMed] [Google Scholar]