Abstract
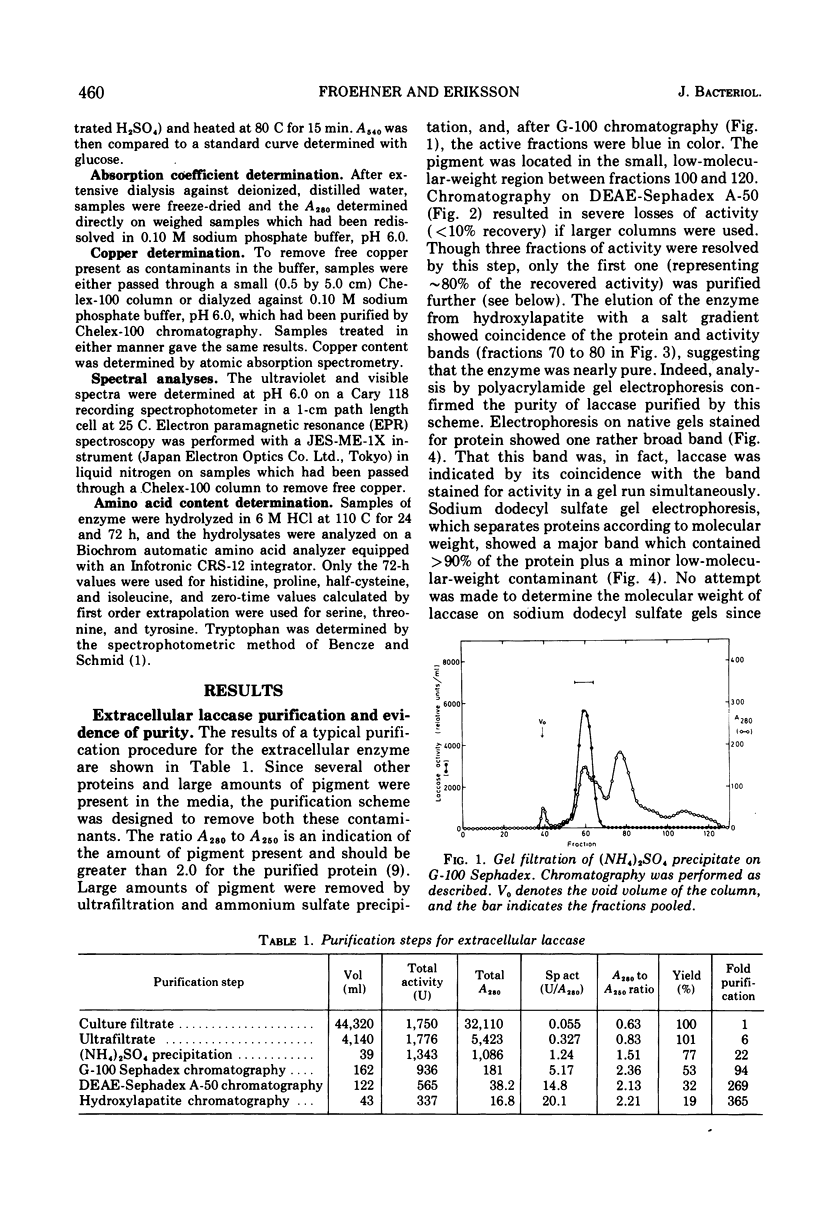
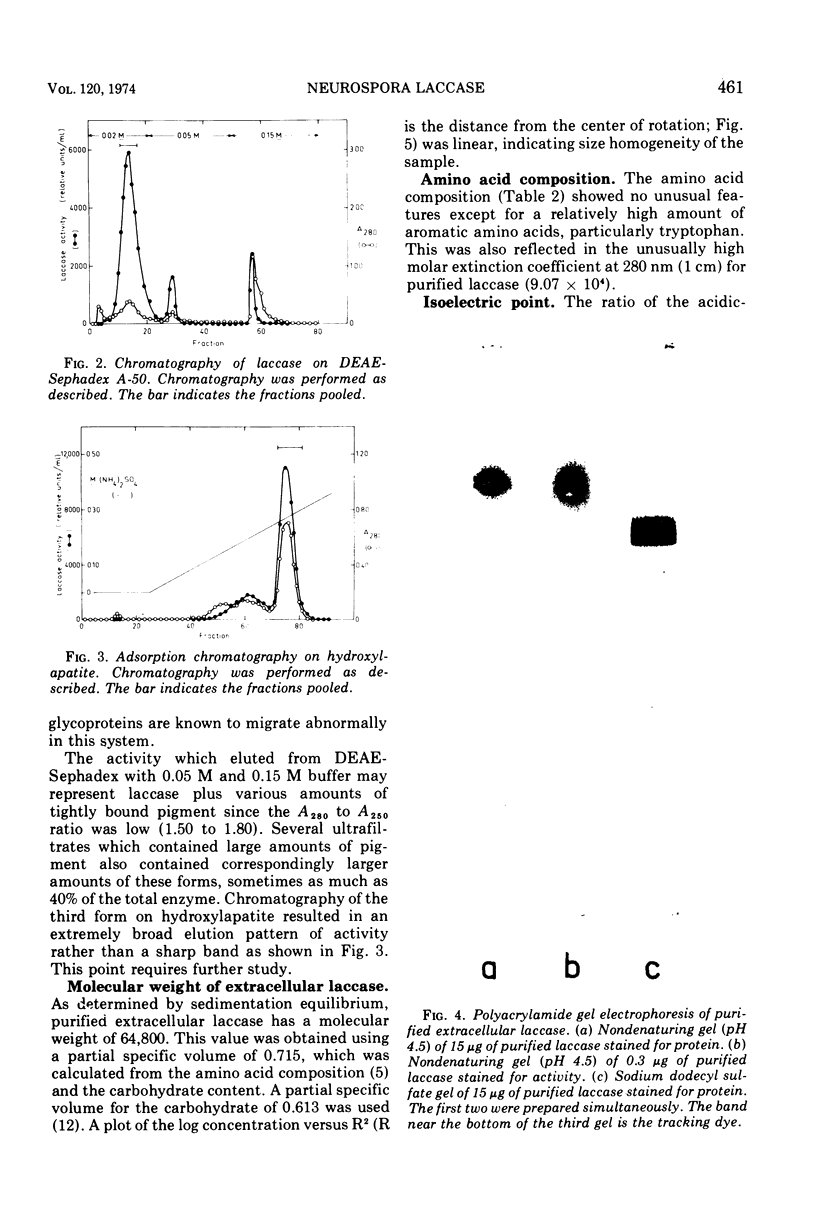
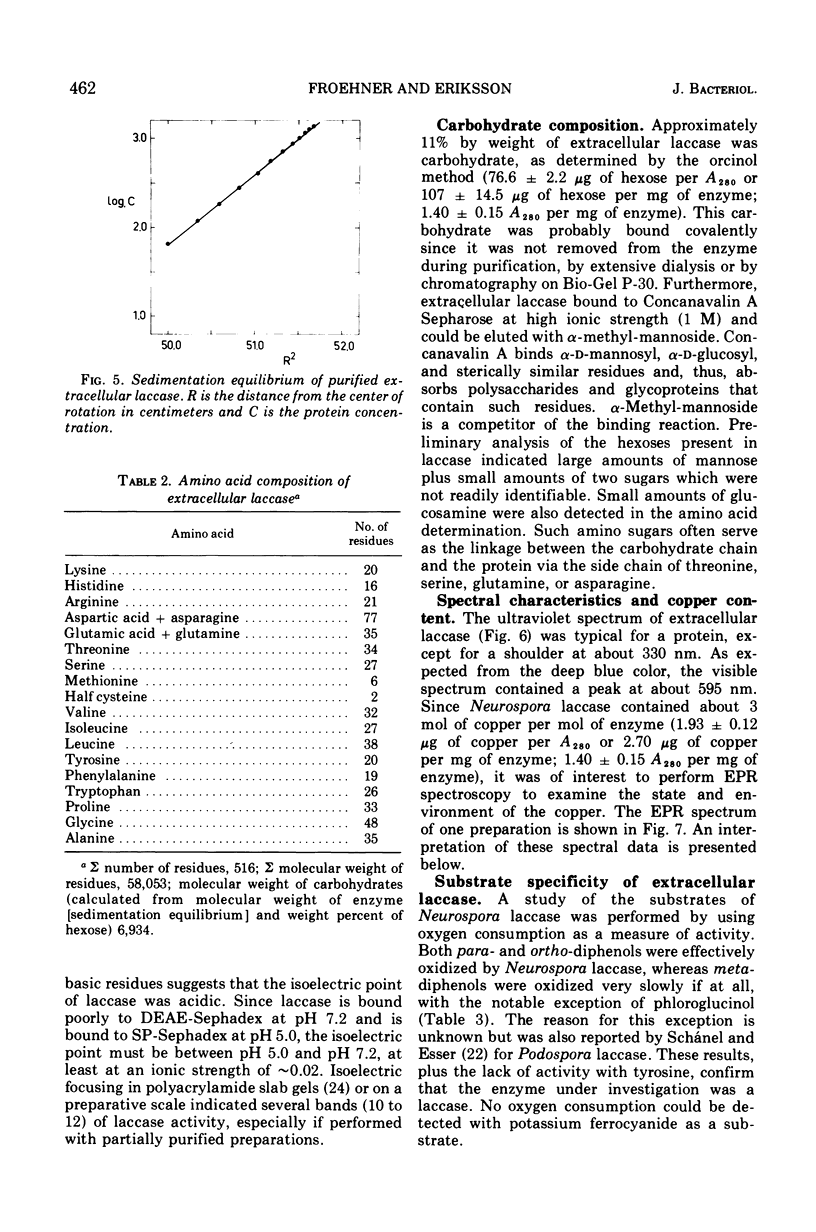
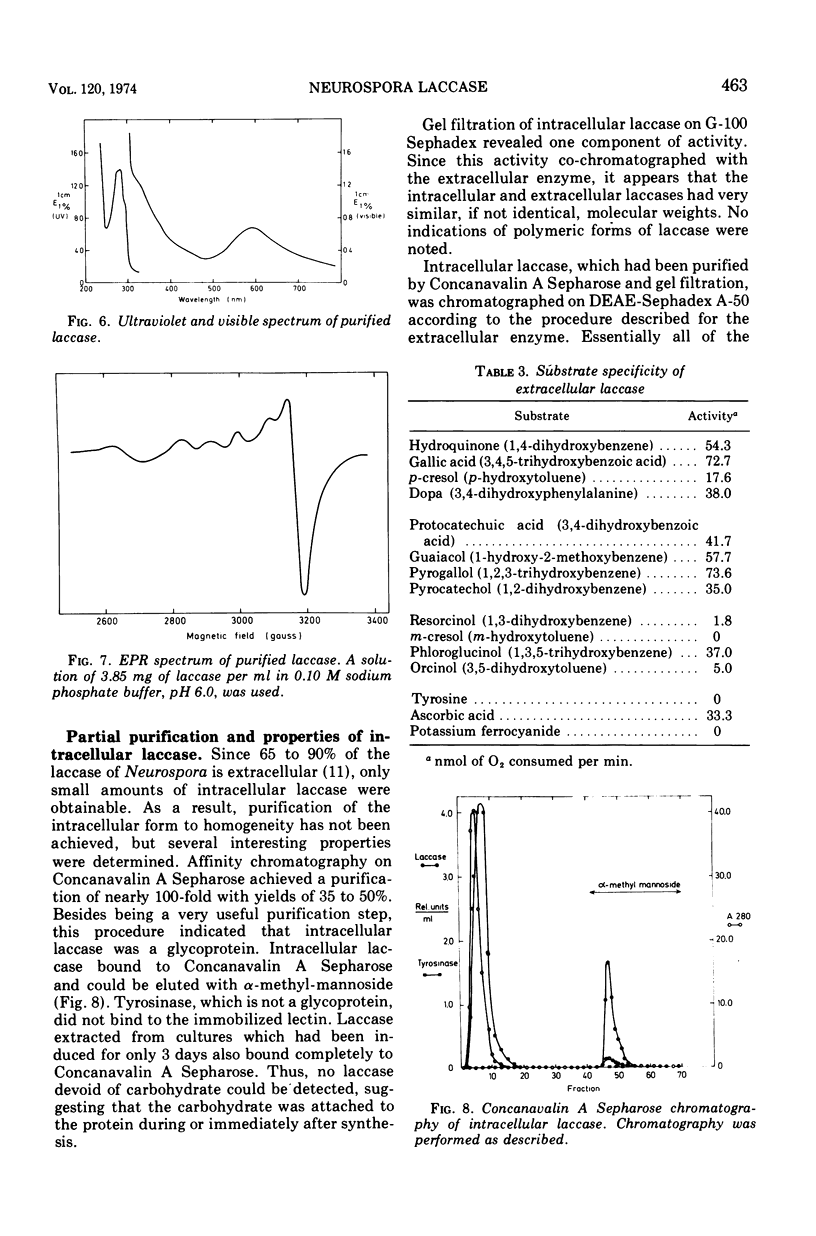
Extracellular Neurospora laccase (p-diphenol:oxygen oxidoreductase; EC 1.10.3.2) has been purified to apparent homogeneity by classical purification techniques. The enzyme, which consists of mainly one form, has a molecular weight of 64,800 and contains 11% carbohydrate. The ultraviolet, visible, and electron paramagnetic resonance spectra indicate that both type I and type II copper are present, as described for the Polyporus versicolor enzyme. With the exception of phloroglucinol, only para- and ortho-diphenols serve as effective substrates for the enzyme. Like the extracellular form, intracellular laccase is a glycoprotein as shown by its ability to bind to Concanavalin A Sepharose. Other studies, including gel filtration and ion-exchange chromatography, revealed no differences between the intracellular and extracellular enzymes, suggesting that intracellular laccase is destined for excretion by the cell.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brändén R., Malmström B. G., Vänngård T. The interaction of fungal laccase with hydrogen peroxide and the removal of fluoride from the inhibited enzyme. Eur J Biochem. 1971 Jan;18(2):238–241. doi: 10.1111/j.1432-1033.1971.tb01236.x. [DOI] [PubMed] [Google Scholar]
- Chervenka C. H. Long-column meniscus depletion sedimentation equilibrium technique for the analytical ultracentrifuge. Anal Biochem. 1970 Mar;34:24–29. doi: 10.1016/0003-2697(70)90082-5. [DOI] [PubMed] [Google Scholar]
- Cheung D. S., Marshall K. C. Antigenic and some kinetic properties of three p-diphenol oxidase isoenzymes of Trametes versicolor. Biochim Biophys Acta. 1969 Mar 18;178(1):177–180. doi: 10.1016/0005-2744(69)90145-4. [DOI] [PubMed] [Google Scholar]
- Esser K., Minuth W. The phenoloxidases of the ascomycete Podospora anserina. Communication 4. Genetic regulation of the formation of laccase. Genetics. 1970 Mar-Apr;64(3):441–458. [PMC free article] [PubMed] [Google Scholar]
- Esser K., Minuth W. The phenoloxidases of the ascomycete Podospora anserina. Microheterogeneity of laccase II. Eur J Biochem. 1971 Dec 10;23(3):484–488. doi: 10.1111/j.1432-1033.1971.tb01644.x. [DOI] [PubMed] [Google Scholar]
- Eylar E. H. On the biological role of glycoproteins. J Theor Biol. 1966 Jan;10(1):89–113. doi: 10.1016/0022-5193(66)90179-2. [DOI] [PubMed] [Google Scholar]
- Farkas V., Svoboda A., Bauer S. Secretion of cell-wall glycoproteins by yeast protoplasts. Effect of 2-deoxy-D-glucose and cycloheximide. Biochem J. 1970 Aug;118(5):755–758. doi: 10.1042/bj1180755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Froehner S. C., Eriksson K. E. Induction of Neurospora crassa laccase with protein synthesis inhibitors. J Bacteriol. 1974 Oct;120(1):450–457. doi: 10.1128/jb.120.1.450-457.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fåhraeus G., Reinhammar B. Large scale production and purification of laccase from cultures of the fungus Polyporus versicolor and some properties of laccase A. Acta Chem Scand. 1967;21(9):2367–2378. doi: 10.3891/acta.chem.scand.21-2367. [DOI] [PubMed] [Google Scholar]
- HOROWITZ N. H., SHEN S. C. Neurospora tyrosinase. J Biol Chem. 1952 May;197(2):513–520. [PubMed] [Google Scholar]
- Jonsson M., Pettersson E., Reinhammar B. Isoelectric fractionation, analysis, and characterization of ampholytes in natural pH gradients. VII. The isoelectric spectra of fungal laccase A and B. Acta Chem Scand. 1968;22(7):2135–2140. doi: 10.3891/acta.chem.scand.22-2135. [DOI] [PubMed] [Google Scholar]
- Kuo S. C., Lampen J. O. Inhibition by 2-deoxy-D-glucose of synthesis of glycoprotein enzymes by protoplasts of Saccharomyces: relation to inhibition of sugar uptake and metabolism. J Bacteriol. 1972 Aug;111(2):419–429. doi: 10.1128/jb.111.2.419-429.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- MOSBACH R. Purification and some properties of laccase from Polyporus versicolor. Biochim Biophys Acta. 1963 Jun 11;73:204–212. doi: 10.1016/0006-3002(63)90304-4. [DOI] [PubMed] [Google Scholar]
- Malmström B. G., Reinhammar B., Vänngård T. Two forms of copper (II) in fungal laccase. Biochim Biophys Acta. 1968 Feb 1;156(1):67–76. doi: 10.1016/0304-4165(68)90105-0. [DOI] [PubMed] [Google Scholar]
- Vesterberg O. Isoelectric focusing of proteins in polyacrylamide gels. Biochim Biophys Acta. 1972 Jan 26;257(1):11–19. doi: 10.1016/0005-2795(72)90248-6. [DOI] [PubMed] [Google Scholar]
- Winterburn P. J., Phelps C. F. The significance of glycosylated proteins. Nature. 1972 Mar 24;236(5343):147–151. doi: 10.1038/236147a0. [DOI] [PubMed] [Google Scholar]




