Abstract
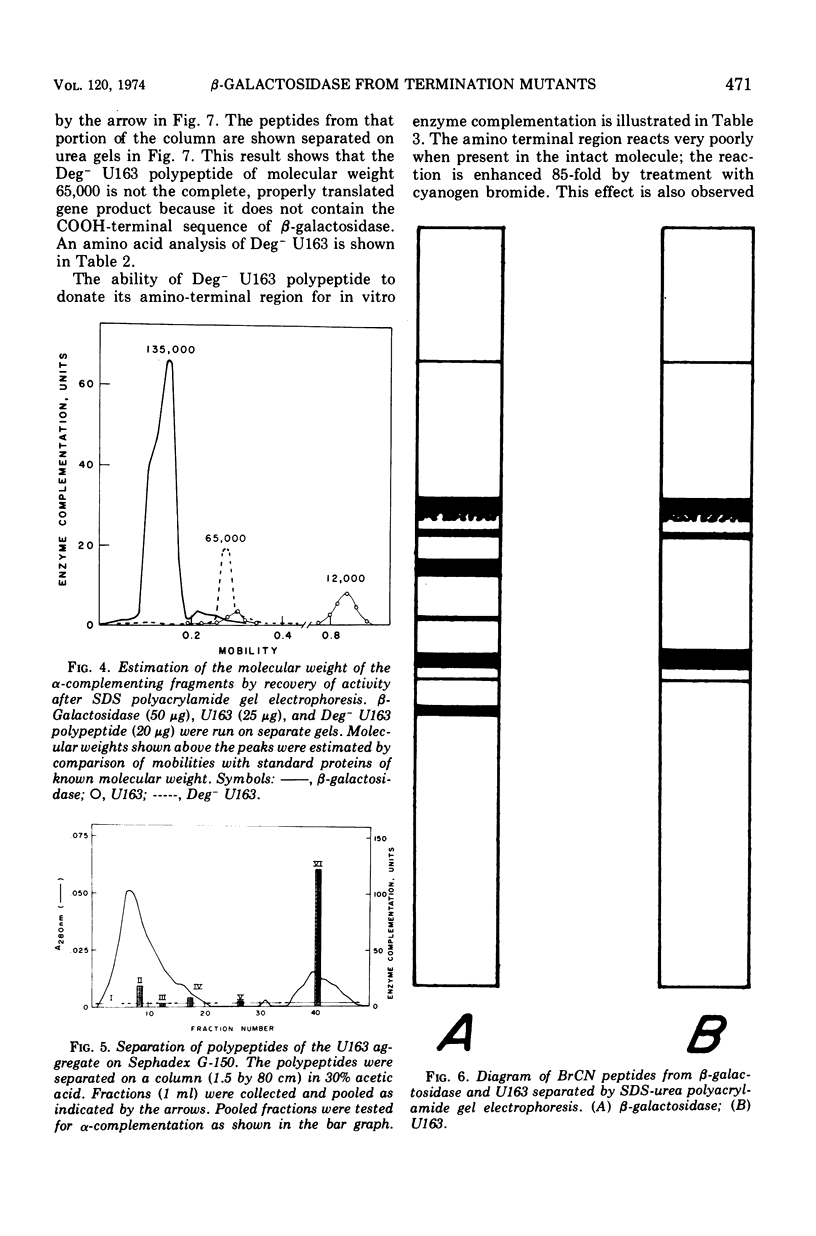
β-Galactosidase fragments were isolated from strains of Escherichia coli with mutations in the lacZ gene. The polypeptide obtained from a termination mutant (lacZNG125) appeared to be the intact gene product, containing the first half of the β-galactosidase amino acid sequence. From an internal deletion mutant strain (lacZU163), an aggregate was obtained of several partially degraded polypeptides. Each of these was smaller than predicted from genetic data for the fragment. Introduction of the lacZU163 mutation into a protein degradation-deficient strain (Deg−) resulted in the protection of the amino-terminal region of the protein. Some of the BrCN peptides from the U163 polypeptides were separated and identified. From such experiments it was shown that in both Deg− and Deg+ strains the COOH-terminal region is rapidly degraded. This indicates that the complete gene product of lacZU163 has not been detected. The use of genetically defined enzyme fragments in studying structure-function relationships and in determination of primary structure is discussed.
Full text
PDF

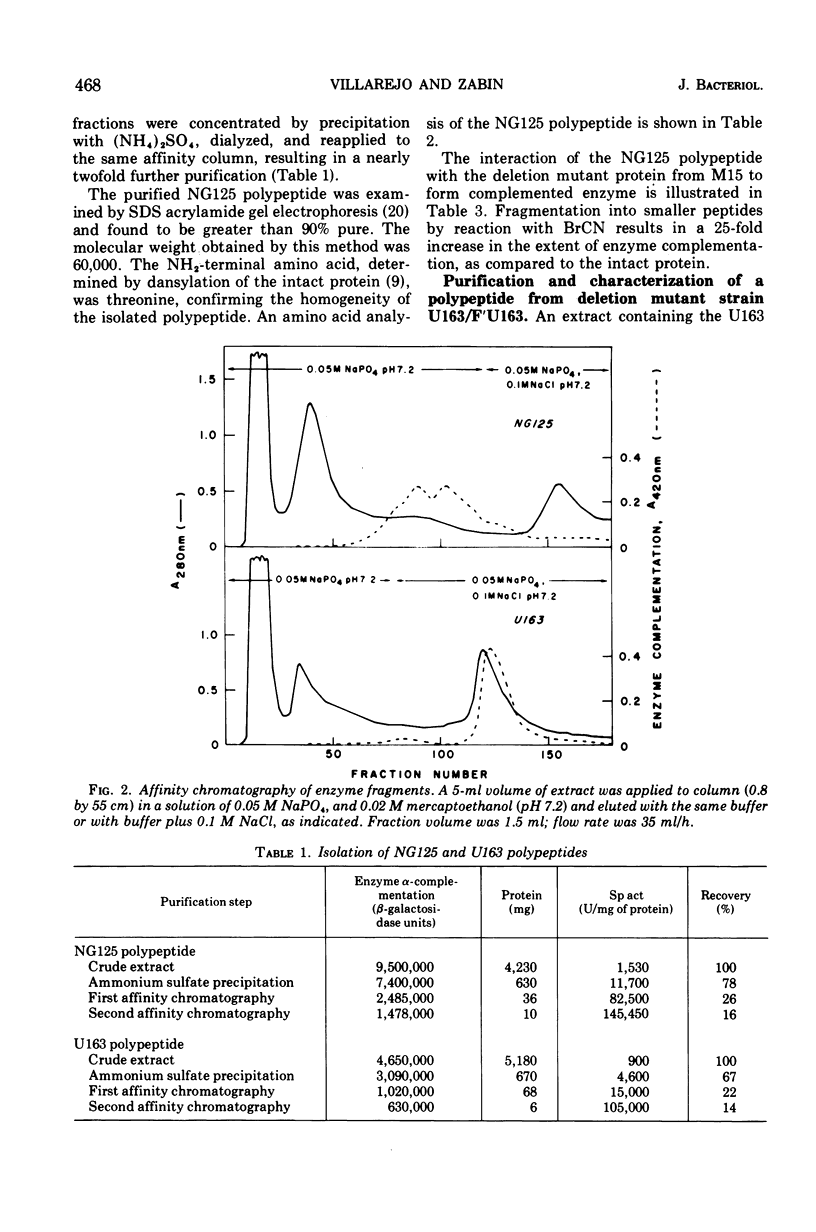

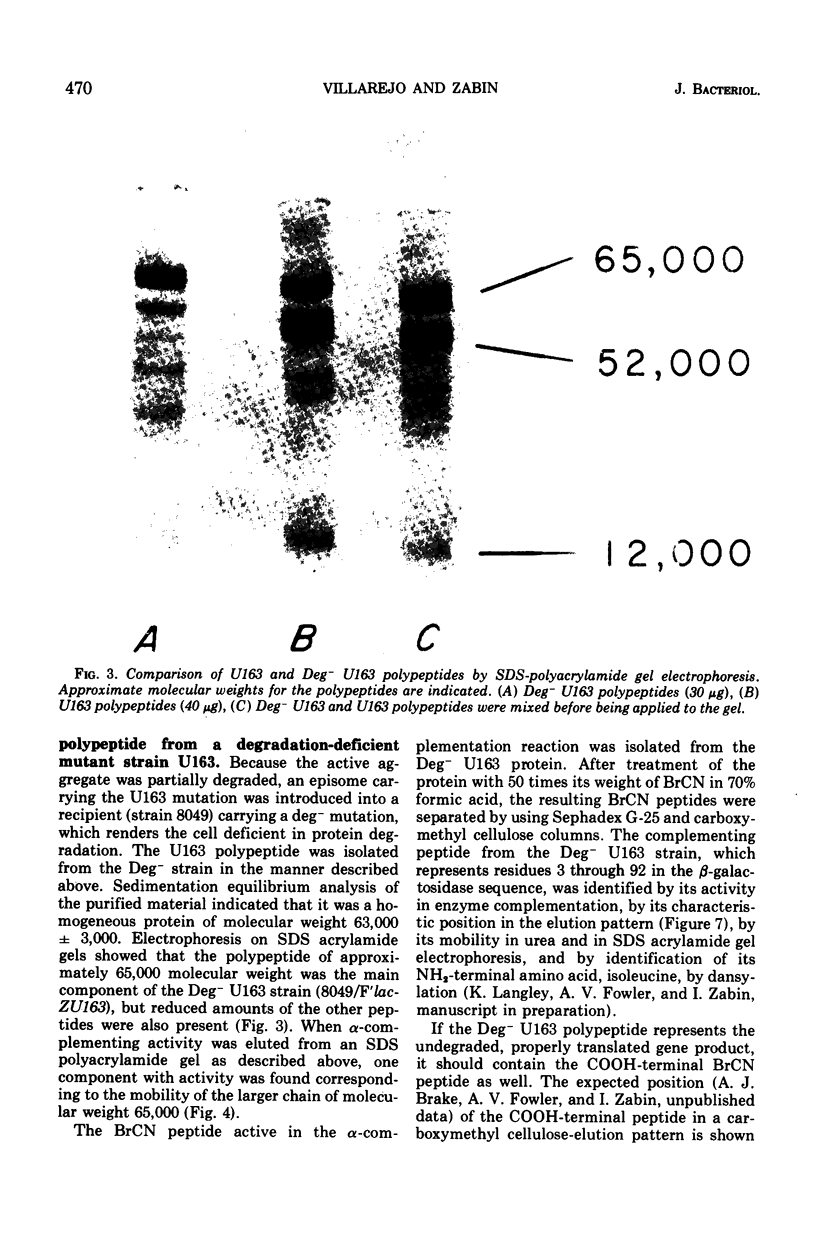



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Berg A. P., Fowler A. V., Zabin I. Beta-galactosidase: isolation of and antibodies to incomplete chains. J Bacteriol. 1970 Feb;101(2):438–443. doi: 10.1128/jb.101.2.438-443.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J. L., Brown D. M., Zabin I. Beta-galactosidase: orientation and the carboxyl-terminal coding site in the gene. Proc Natl Acad Sci U S A. 1967 Sep;58(3):1139–1143. doi: 10.1073/pnas.58.3.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukhari A. I., Zipser D. Mutants of Escherichia coli with a defect in the degradation of nonsense fragments. Nat New Biol. 1973 Jun 20;243(129):238–241. doi: 10.1038/newbio243238a0. [DOI] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. Beta-galactosidase: immunological studies of nonsense, missense and deletion mutants. J Mol Biol. 1968 Apr 14;33(1):35–47. doi: 10.1016/0022-2836(68)90279-9. [DOI] [PubMed] [Google Scholar]
- Fowler A. V., Zabin I. The amino acid sequence of beta galactosidase. I. Isolation and composition of tryptic peptides. J Biol Chem. 1970 Oct 10;245(19):5032–5041. [PubMed] [Google Scholar]
- Goldschmidt R. In vivo degradation of nonsense fragments in E. coli. Nature. 1970 Dec 19;228(5277):1151–1154. doi: 10.1038/2281151a0. [DOI] [PubMed] [Google Scholar]
- HORIUCHI T., TOMIZAWA J. I., NOVICK A. Isolation and properties of bacteria capable of high rates of beta-galactosidase synthesis. Biochim Biophys Acta. 1962 Jan 22;55:152–163. doi: 10.1016/0006-3002(62)90941-1. [DOI] [PubMed] [Google Scholar]
- JOVIN T., CHRAMBACH A., NAUGHTON M. A. AN APPARATUS FOR PREPARATIVE TEMPERATURE-REGULATED POLYACRYLAMIDE GEL ELECTROPHORESIS. Anal Biochem. 1964 Nov;9:351–369. doi: 10.1016/0003-2697(64)90192-7. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lin S., Zabin I. Beta-galactosidase. Rates of synthesis and degradation of incomplete chains. J Biol Chem. 1972 Apr 10;247(7):2205–2211. [PubMed] [Google Scholar]
- Newton W. A., Beckwith J. R., Zipser D., Brenner S. Nonsense mutants and polarity in the lac operon of Escherichia coli. J Mol Biol. 1965 Nov;14(1):290–296. doi: 10.1016/s0022-2836(65)80250-9. [DOI] [PubMed] [Google Scholar]
- Steers E., Jr, Cuatrecasas P., Pollard H. B. The purification of beta-galactosidase from Escherichia coli by affinity chromatography. J Biol Chem. 1971 Jan 10;246(1):196–200. [PubMed] [Google Scholar]
- Swank R. T., Munkres K. D. Molecular weight analysis of oligopeptides by electrophoresis in polyacrylamide gel with sodium dodecyl sulfate. Anal Biochem. 1971 Feb;39(2):462–477. doi: 10.1016/0003-2697(71)90436-2. [DOI] [PubMed] [Google Scholar]
- Villarejo M. R., Zabin I. Affinity chromatography of -galactosidase fragments. Nat New Biol. 1973 Mar 14;242(115):50–52. doi: 10.1038/newbio242050a0. [DOI] [PubMed] [Google Scholar]
- Villarejo M., Zamenhof P. J., Zabin I. Beta-galactosidase. In vivo -complementation. J Biol Chem. 1972 Apr 10;247(7):2212–2216. [PubMed] [Google Scholar]
- Weber K., Osborn M. The reliability of molecular weight determinations by dodecyl sulfate-polyacrylamide gel electrophoresis. J Biol Chem. 1969 Aug 25;244(16):4406–4412. [PubMed] [Google Scholar]
- Zabin I., Fowler A. V. The amino acid sequence of -galactosidase. 3. The sequences of NH 2 - and COOH-terminal tryptic peptides. J Biol Chem. 1972 Sep 10;247(17):5432–5435. [PubMed] [Google Scholar]
- Zamenhof P. J., Villarejo M. Construction and properties of Escherichia coli strains exhibiting -complementation of -galactosidase fragments in vivo. J Bacteriol. 1972 Apr;110(1):171–178. doi: 10.1128/jb.110.1.171-178.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]