Abstract
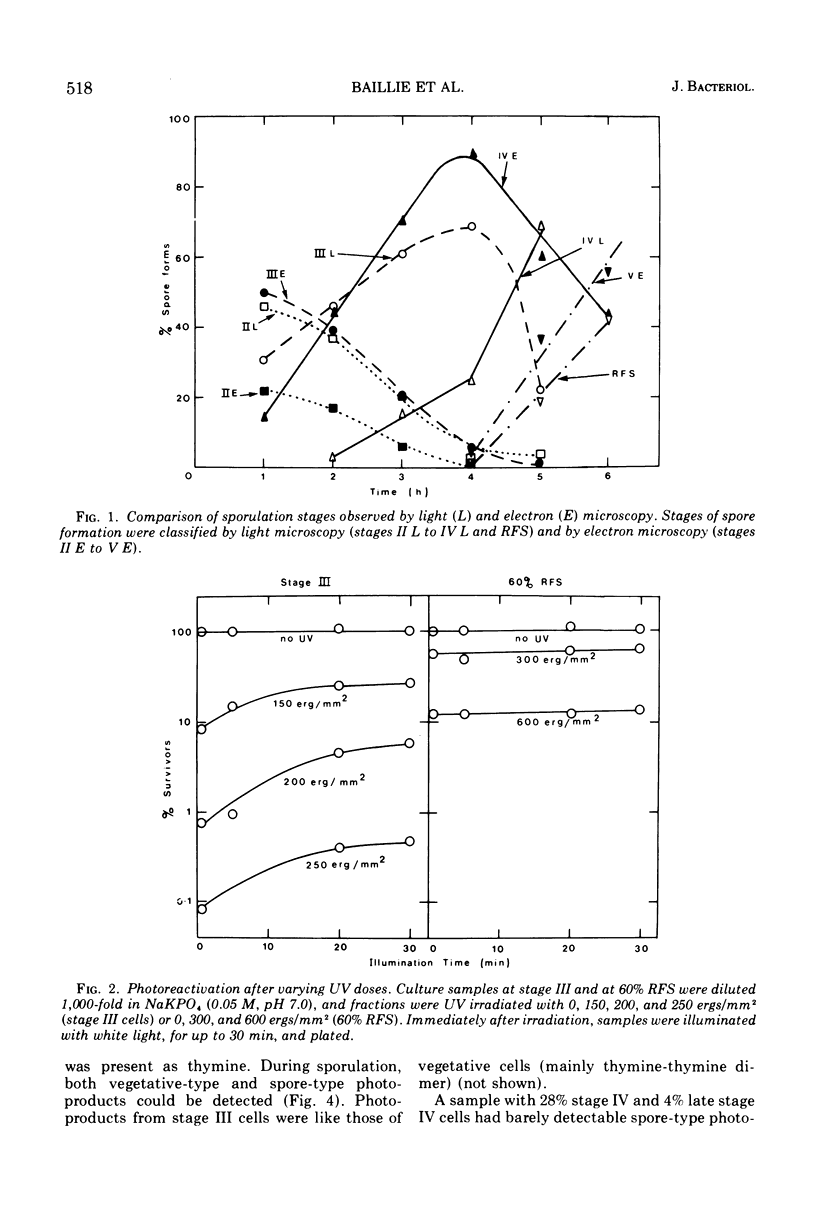
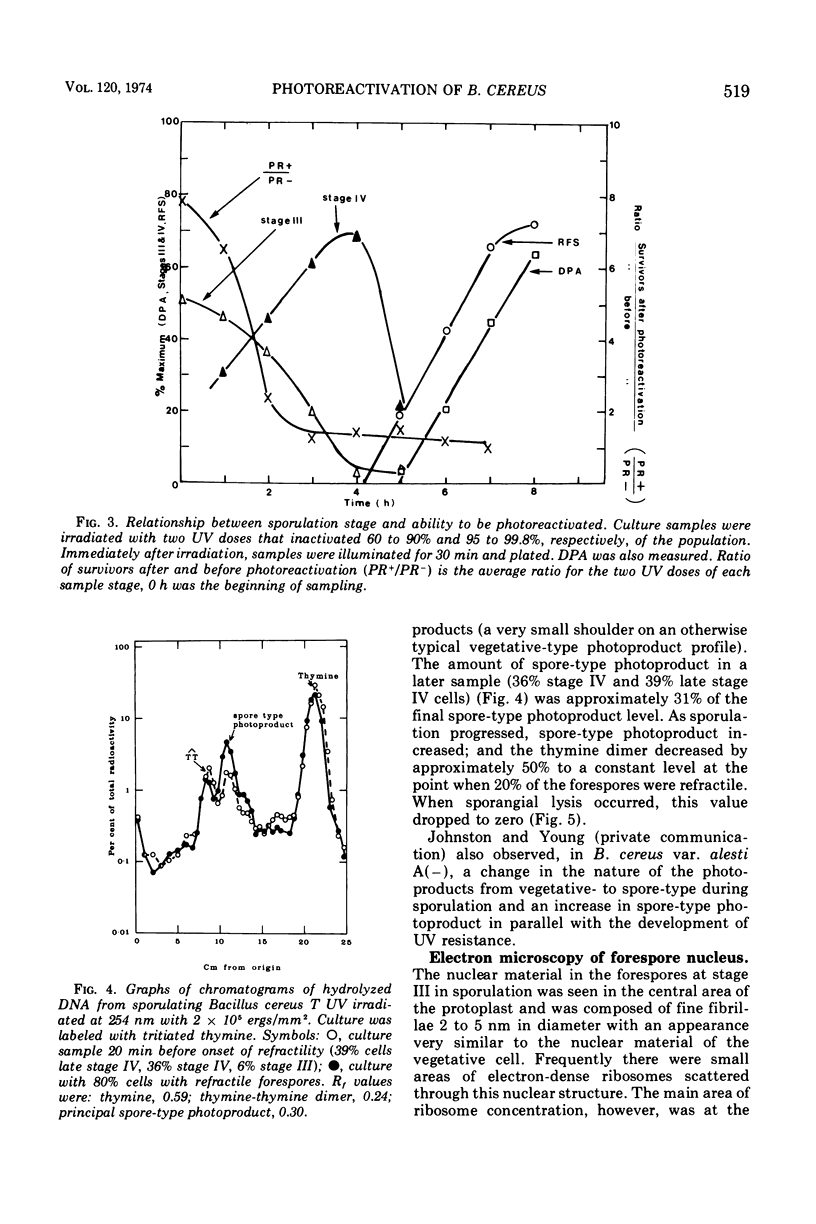
Photoreactivation of ultraviolet-irradiated Bacillus cereus T declined markedly during the development of stage IV forespores. During ultraviolet irradiation of a culture containing early and late stage IV forespores, both vegetative- and spore-type photoproducts were formed. The formation of vegetative-type photoproducts (mainly thymine dimers) decreased to nearly half during late stage IV, remaining constant until lysis of the mother cells began, when it fell to zero. Spore-type photoproducts were first observed during late stage IV and increased with the increase in numbers of late stage IV forespores. The occurrence of spore-type photoproducts preceded the development of refractile forespores by about 1 h. At stage III the nuclear material occupied a central position, and the ribosomes were at the periphery of the forespore protoplast. During stage IV the deoxyribonucleic acid (DNA) occurred in a peripheral position, and bundles of fibers (“transition” DNA) could be seen. By stage V, all of the DNA appeared to be of the spore type and was peripheral, and the forespore protoplast center was packed with ribosomes. Forespore stages II, III, and IV were classified by light and electron microscopy. The curve for electron microscope classifications preceded that for light microscope classifications by approximately one stage. The formation of spore-type photoproducts preceded differentiation of DNA by about 1 h, the latter coinciding with the development of refractility. Spore-type photoproducts have been associated with DNA in the A state, and the progressive change of the forespore DNA into this state is discussed in relation to the spore differentiation process.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beabealashvily R. S., Ivanov V. I., Minchenkova L. E., Savotchkina L. P. RNA polymerase-DNA complexes. I. The study of the conformation of nucleic acids at the growing point of RNA in an RNA polymerase-DNA system. Biochim Biophys Acta. 1972 Jan 18;259(1):35–40. doi: 10.1016/0005-2787(72)90471-6. [DOI] [PubMed] [Google Scholar]
- Cooper P. J., Hamilton L. D. The A-B conformational change in the sodium salt of DNA. J Mol Biol. 1966 Apr;16(2):562–563. doi: 10.1016/s0022-2836(66)80193-6. [DOI] [PubMed] [Google Scholar]
- Coote J. G., Mandelstam J. Use of constructed double mutants for determining the temporal order of expression of sporulation genes in Bacillus subtilis. J Bacteriol. 1973 Jun;114(3):1254–1263. doi: 10.1128/jb.114.3.1254-1263.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnellan J. E., Jr, Setlow R. B. Thymine Photoproducts but not Thymine Dimers Found in Ultraviolet-Irradiated Bacterial Spores. Science. 1965 Jul 16;149(3681):308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- Donnellan J. E., Jr, Stafford R. S. The ultraviolet photochemistry and photobiology of vegetative cells and spores of Bacillus megaterium. Biophys J. 1968 Jan;8(1):17–28. doi: 10.1016/S0006-3495(68)86471-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fréhel C., Ryter A. Réversibilité de la sporulation chez B. subtilis. Ann Inst Pasteur (Paris) 1969 Sep;117(3):297–311. [PubMed] [Google Scholar]
- Fuller W. Molecular structure and biological function. Adv Sci. 1970 Mar;26(129):267–288. [PubMed] [Google Scholar]
- Germaine G. R., Coggiola E., Murrell W. G. Development of ultraviolet resistance in sporulating Bacillus cereus T. J Bacteriol. 1973 Nov;116(2):823–831. doi: 10.1128/jb.116.2.823-831.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germaine G. R., Murrell W. G. Effect of dipicolinic acid on the ultraviolet radiation resistance of Bacillus cereus spores. Photochem Photobiol. 1973 Mar;17(3):145–153. doi: 10.1111/j.1751-1097.1973.tb06344.x. [DOI] [PubMed] [Google Scholar]
- Germaine G. R., Murrell W. G. Use of ultraviolet radiation to locate dipicolinic acid in Bacillus cereus spores. J Bacteriol. 1974 Apr;118(1):202–208. doi: 10.1128/jb.118.1.202-208.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon R. A., Murrell W. G. Simple method of detecting spore septum formation and synchrony of sporulation. J Bacteriol. 1967 Jan;93(1):495–496. doi: 10.1128/jb.93.1.495-496.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KELLENBERGER E., RYTER A., SECHAUD J. Electron microscope study of DNA-containing plasms. II. Vegetative and mature phage DNA as compared with normal bacterial nucleoids in different physiological states. J Biophys Biochem Cytol. 1958 Nov 25;4(6):671–678. doi: 10.1083/jcb.4.6.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leanz G., Gilvarg C. Dipicolinic acid location in intact spores of Bacillus megaterium. J Bacteriol. 1973 Apr;114(1):455–456. doi: 10.1128/jb.114.1.455-456.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis J. C. Determination of dipicolinic acid in bacterial spores by ultraviolet spectrometry of the calcium chelate. Anal Biochem. 1967 May;19(2):327–337. doi: 10.1016/0003-2697(67)90168-6. [DOI] [PubMed] [Google Scholar]
- ROMIG W. R., WYSS O. Some effects of ultraviolet radiation of sporulating cultures of Bacillus cereus. J Bacteriol. 1957 Sep;74(3):386–391. doi: 10.1128/jb.74.3.386-391.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahn R. O., Hosszu H. L. Influence of relative humidity on the photochemistry of DNA films. Biochim Biophys Acta. 1969 Sep 17;190(1):126–131. doi: 10.1016/0005-2787(69)90161-0. [DOI] [PubMed] [Google Scholar]
- Smith K. C., Yoshikawa H. Variation in the photochemical reactivity of thymine in the DNA of B. subtilis spores, vegetative cells and spores germinated in chloramphenicol. Photochem Photobiol. 1966 Oct;5(10):777–786. doi: 10.1111/j.1751-1097.1966.tb05773.x. [DOI] [PubMed] [Google Scholar]
- Stafford R. S., Donnellan J. E., Jr Photochemical evidence for conformation changes in DNA during germination of bacterial spores. Proc Natl Acad Sci U S A. 1968 Mar;59(3):822–828. doi: 10.1073/pnas.59.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varghese A. J. 5-Thyminyl-5,6-dihydrothymine from DNA irradiated with ultraviolet light. Biochem Biophys Res Commun. 1970 Feb 6;38(3):484–490. doi: 10.1016/0006-291x(70)90739-4. [DOI] [PubMed] [Google Scholar]



